- US 4595688 Certain Hexahydro-6-Arylprylpyrrolo [2,1-A]Isoquinoline
- US 4837328 Stereoselective reaction for hexahydro-6-arylpyrrolo(2,1-A) isoquinolines
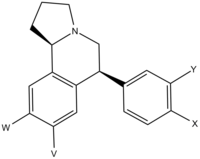
| X | Y | V | W | MA (mg/kg) | ptosis (mg/kg) | DA (nM) | NE (nM) | 5-HT (nM) |
| H | H | H | H | 0.34 (0.59) | 0.07 (0.05) | 11.3 (4.4) | 0.60 (0.37) | 23.5 (12.4) |
| H | H | OMe | OMe | 15.1 | 3.8 | 15.0 | 53.7 | 1540 |
| H | H | OH | OH | 0.87 | 0.53 | 43.5 | 10.5 | 124 |
| OMe | H | H | H | 0.27 | 0.03 | 5.2 | 0.79 | 1.7 |
| OH | H | H | H | 0.40 | 0.09 | 5.1 | 0.74 | 3.2 |
| H | OMe | H | H | ~0.2 | 0.07 | 15.8 | 0.65 | 7.2 |
| H | OH | H | H | >10 | 0.11 | 10.1 | 0.85 | 24.6 |
| H | H | H | OMe | no data | no data | 2.8 | 2.2 | 4.5 |
| OMe | OMe | H | H | 2.0 | 0.13 | 71.9 | 3.4 | 18.1 |
| OH | OH | H | H | 0.19 | 0.11 | 10.1 | 0.81 | 33.1 |
| Cl | H | H | H | 0.55 | 0.34 | 1.7 | 0.16 | 1.5 |
| H | Cl | H | H | ~0.1 | <0.1 | 2.5 | 0.45 | 7.3 |
| Cl | H | H | Cl | 37.4 | ~4 | 3.2 | 3.2 | 2.9 |
| Cl | Cl | H | H | 0.39 | 0.14 | 0.99 | 0.68 | 1.8 |
| F | H | H | H | ~0.2 | ~0.2 | 8.4 | 1.4 | 8.5 |
| F | H | H | F | >30 | 0.05 | 7.7 | 0.55 | 4.4 |
| NH2 | H | H | H | ~0.2 | ~0.01 | 0.86 | 0.20 | 44 |
| SMe | H | H | H | >30 (no data) | 0.30 (no data) | 41.2 (23.5) | 3.0 (1.8) | 0.62 (0.39) |
| Ethynyl | H | H | H | ~0.5 | ~0.5 | 2.6 | 0.94 | 1.0 |
| diclofensine | | | 10.9 | 8.8 | 10.3 | | | |
| WIN-25978 | | | 7.2 | 41.1 | 879 | | | |
I don't think it takes a genius to note that the above compounds are all derived from the amphetamine scaffold. Note that the p-SCH3 is selective to SERT (like 4MTA), p-OCH3 similar but less so (like p-methoxy amphetamine), the m-methoxy more serotonin-selective & the p-Cl is fairly balanced (like p-Cl amphetamine).
What firmly is NOT mentioned is the homologue with the 3,4-methylenedioxy ring or the p-Me, m-OCH3. But given their affinity and LogP, this scaffold might well prove to provide an MDMA-like entactogen with the simple advantage of being at least an order of magnitude more potent. Not easily made, but not controlled.
It's also worth noting that the p,m-dichloro derivative is potent and it makes me wonder what happens to the unwanted enantiomers of sertraline, especially since an earlier antidepressant developed by a Swedish team lacked the 3,4-dichloro and while active, wasn't marketed because it produced euphoria.
If you read the patents to see who THEY reference, you will discover that ALL stimulants are covered thus:
(ring substituted) amphetamines --> (ring substituted) desoxypipradrol (and homologues) --> nomifensine/dichlofensine (and homologues) and the onwards to the scaffold shown above (McN5652/JWJ7925476) and homologues.
I feel it's of value to those seeking to build a training-set. Their are certainly enough scaffolds for one to produce relative spatail positions for the RA (ring aromatics), PI (positively ionizable function) and HBOs (hydrogen-bond acceptors).