-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
25B-NB (n-Benzyl-2C-B)
- Thread starter Dextrose
- Start date
MurphyClox
Bluelighter
- Joined
- Mar 26, 2008
- Messages
- 1,416
I have the same feeling with lots of threads here at ADD.I have a constant feeling people do not read the whole thread before posting.

REALLY NICE WORK Erny! Keep it up, man!
To answer this, I quote a post from Erny (from the dark side of the force):Is Phe339 involved in π-π interactions with the planar aryl component of ergolines, tryptamines and phenethylamines? Without the phenyl ring all these lose activity.
There are no aromatic amino acids for п-stacking in Nichols model as well, although it is evident from mutagenesis studies, that there should be some interaction between Phe 340 (222) and phenylethylamine molecules that is vial for their activity.
- Murphy
Last edited:
IGNVS
Bluelighter
sorry, its alot of info to take in. i dont read the whole thread every time i post and it had been a while since it got bumped back up. my bad.
so basically what im getting from that is that it dosnt matter whats stuck on there (carbonyl or methoxy, hydroxy etc...) right?
so basically what im getting from that is that it dosnt matter whats stuck on there (carbonyl or methoxy, hydroxy etc...) right?
It certainly does, it depends on the type of hydrogen bond that is possible with the appropriate amino acid residue, possible orientations of electron pairs in the substituent and residue atoms, steric hindrance and such. Wer'e talking about 5-HT2A receptor topology.IGNVS said:so basically what im getting from that is that it dosnt matter whats stuck on there (carbonyl or methoxy, hydroxy etc...) right?
There is an answer to your question about 2-methoxy to 2-carboxamido (or 2-(N,N-diethylcarboxamido)) replacement in Braden's dissertation, N-(2-carboxamidobenzyl)-2C-I affinity is lower than that of N-benzyl-2C-I.
If there is no interaction with Asp 343, while interaction with Phe 339 is still a must, one possible conformation for NBOMe phenylethylamines is such that 2-methoxybenzyl oxygen forms a hydrogen bond with Tyr 371 hydroxyl proton, plus additionally hydrogen of the 2-hydroxybenzyl OH group may form a hydrogen bond with Asp 155 oxygen in NBOH phenylethylamines. The latter residue always forms an ionic bond with the amino functions of the ligands. There is also a hydrophobic interaction with valine 367, and п-п interaction with Phe 339 seem to be of the "edge-face" type (T-shape).

This conformation does not conflict with the known SAR's as far as I can see, but has no strong supporting data behind it and thus can also be invalid. This is also the exact conformation I've obtained for the more rigid NBOMe-TCB-2 molecule after energy minimisation, only 5-methoxy of it's base phenyl (where bromine atom is) should point downwards (at the intracellular side) like in binding orientation proposed for 2C-X and DOX molecules by Glennon. On the image of NBOH-2C-C above it is pointing upwards (to the extracellular side), like in models proposed by Nichols.
I'll quote Braden's dissertation more
Michael Robert Braden said:The trends observed in these figures seem to indicate that both F6.51(339) and F6.52(340) are interacting with the N-Benzyls, whereas F6.52(340) appears to be interacting mainly with N-unsubstituted phenethylamines and the other classic agonists. The ∆∆G° values of about 2-4 kcal/mol and centroid-centroid distances of about 4.6 to 4.9Å are consistent with the energy and orientation of a “T-shape” π-π interaction (Jorgensen and Severance, 1990).
...
These results seem to indicate that an interaction of N6.55(343) with ergolines or N-Benzyls does not occur, or is not critical for binding and/or activity. There seems to be some indication that ring-substituted tryptamines may be able to adopt an orientation alternate to the conventional view in the agonist binding site so they can interact with N6.55(343). One could imagine the indole ring “flipping over” so that the indole nitrogen hydrogen would interact with the carbonyl oxygen of N6.55(343). Similarly, mescaline and its analogues may be adopting alternate or additional orientations that allow them to interact with this residue. Therefore, additional experiments with N(1)- substituted tryptamines at this mutant receptor may be necessary. Alternately, it is possible that N6.55(343) is an anchoring residue for the extracellular loop connecting TM4 and TM5 (EL2). The “capping” of the receptor by EL2 may alter ligand signaling or bring other receptor residues into contact with the ligand and could be altered by the N6.55(343)A mutant. This possibility is very difficult to model due to lack of accurate solvation of these residues in our work.
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
I think the above still leaves the inductive effect-potency reversal associated with the n-benzyloxy compounds compared to the classic phenethylamine unaddressed.
You might want to give the modelling a go in CHARMM.
I'll venture a guess to circumvent the liver destruction: replace the oxygen with a nitrogen and then cyclize it with the methyl group on the benzylamino ring to form a pyrrolidine ring. With the free rotation about the amine it should still be able to bind effectively as well as participate in all the hydrogen bonding. It would also confirm your binding profile (I think). Even the tetrahydrofuran analogue should work if the 2-methoxy variants are very active.
You might want to give the modelling a go in CHARMM.
I'll venture a guess to circumvent the liver destruction: replace the oxygen with a nitrogen and then cyclize it with the methyl group on the benzylamino ring to form a pyrrolidine ring. With the free rotation about the amine it should still be able to bind effectively as well as participate in all the hydrogen bonding. It would also confirm your binding profile (I think). Even the tetrahydrofuran analogue should work if the 2-methoxy variants are very active.
Attachments
Last edited:
realgar
Bluelighter
- Joined
- Jan 22, 2007
- Messages
- 45
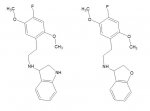
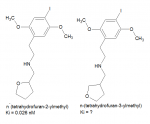
What intrigues me is the actual structure of the substance with the highest affinity for h5-HT2A receptors (0.026 nM vs. 0.044 nM for 25I-NBOMe) in Braden's dissertation.
It is called 25I-NBDHF in Table A.1 (p. 151), or 25I-NDHF (one letter missing) in Figure A.1 (p. 147). Both abbreviations don't correspond to the structure shown, which is 2C-I-N-tetrahydrofurfuryl (or tetrahydrofuran-2-ylmethyl).
It is called 25I-NBDHF in Table A.1 (p. 151), or 25I-NDHF (one letter missing) in Figure A.1 (p. 147). Both abbreviations don't correspond to the structure shown, which is 2C-I-N-tetrahydrofurfuryl (or tetrahydrofuran-2-ylmethyl).
nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
Here are pretty pictures of that compound and another analogue.
Between the Ki data for both the n-(2-methoxybenzyl) and n-(tetrahydrofuran-2-ylmethyl) substituent, as well as the structural data pointed out by Erny, I feel as if that the benzofuran/pyrrolidine I had proposed may well be extremely potent.
Between the Ki data for both the n-(2-methoxybenzyl) and n-(tetrahydrofuran-2-ylmethyl) substituent, as well as the structural data pointed out by Erny, I feel as if that the benzofuran/pyrrolidine I had proposed may well be extremely potent.
Attachments
realgar
Bluelighter
- Joined
- Jan 22, 2007
- Messages
- 45
Can I get more information (citation?) for that Ralf Heim dissertation?
Here it is.
_http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221
Here it is.
_http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221
Thank you so much.
aloha
psilo
SBASE_MAN
Greenlighter
SWIM snorted what they were told was 2cb and the effects were impressive, however through browsing on the internet i found the half life of 2cb should be no more than 4-5 days, it has been 7 days and last night SWIM's heartrate went to 128 bpm, SWIM complianed of shooting pains in the back of their arm, and a crushing pain between in the centre of their chest between the ribs, so because they were feeling similar symptoms to a heart attack called the paramedics, and was promptly taken to hospital- blood pressure was low, legs were shakey, nausiating feeling, very fast resting heart rate which kept between 104 and 118 for 5 minutes solid.
The trip SWIM had was good, when it wasnt a battle between a good and bad trip.
2 days after SWIM took anfetimine, this brought on panic attacks, unsettled breathing etc.
Having been to the Hospital SWIM's physiology appeared normal,
so i wonder if the type of bad delayed trip you can get on mushrooms and LSD can also exist in a drug with a chemical with a supposedly short half life. Or could it be psychosymatic?
The trip SWIM had was good, when it wasnt a battle between a good and bad trip.
2 days after SWIM took anfetimine, this brought on panic attacks, unsettled breathing etc.
Having been to the Hospital SWIM's physiology appeared normal,
so i wonder if the type of bad delayed trip you can get on mushrooms and LSD can also exist in a drug with a chemical with a supposedly short half life. Or could it be psychosymatic?
IGNVS
Bluelighter
so any new info on this stuff, new trip reports, chems, etc?
dread
Bluelighter
http://en.wikipedia.org/wiki/2CBFly-NBOMe
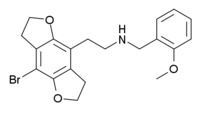
http://en.wikipedia.org/wiki/25I-NBMD
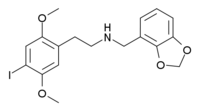
If anyone has any information about these please post... particularly the first one seems very interesting.

http://en.wikipedia.org/wiki/25I-NBMD

If anyone has any information about these please post... particularly the first one seems very interesting.
dorothyperkins
Bluelighter
- Joined
- Oct 18, 2007
- Messages
- 426
Have Ki's ([125I]-DOI):
2CBFly-NBOMe - r5-HT2A 0.14 nm, r5-HT2C 0.26 nm
25I-NBMD- r5-HT2A 0.193 nm, r5-HT2C 0.41 nm
h5-HT2A 0.049 nm, h5-HT2C 1.70 nm
I'm more interested in the second one, it has 35-fold greater affinity for the h5-HT2A receptor than the 2C receptor.
2CBFly-NBOMe - r5-HT2A 0.14 nm, r5-HT2C 0.26 nm
25I-NBMD- r5-HT2A 0.193 nm, r5-HT2C 0.41 nm
h5-HT2A 0.049 nm, h5-HT2C 1.70 nm
I'm more interested in the second one, it has 35-fold greater affinity for the h5-HT2A receptor than the 2C receptor.
rakketakke
Bluelighter
Has anybody have any more information by now?
amanitadine
Bluelighter
Uhhh.... as to N-Benzyl-2C-B or N-Benzyl PEAs in general? Do shitloads of feckless in-vitro tests and sales on the open market qualify as more information?  The cat is out of the bag on these......should be interesting
The cat is out of the bag on these......should be interesting

Last edited:
tryp2fun
Bluelighter
- Joined
- Dec 24, 2008
- Messages
- 253
With all the hubbub about the NBOMs these days, its time to bump this thread. As expected, yes, 25B-NB is an active psychedelic. Like the NBOMs, it is inactive orally, but it is active sublingually, with threshold effects found at 4 mg and full effects at 12-14 mg. One experiment with 6 mg insufflated also was active, comparable to 10-12 mg sublingually. The effects are similar to 2C-B, but the pharmacokinetics are different. Sublingually, the first alert is at about 20 minutes, with the peak reached at around 45 minutes to an hour, lasting only about an hour, then a rapid decline to baseline at around 3 hours.
ebola?
Bluelight Crew
Careful guys:
the identity and purity of this compound is open to question. I would begin with lower doses than these (in the range of NBOMe substituted 2Cs) until more reports flow in.
...
It would be really interesting if this were to pan out though, as it would begin to map the SAR of this class of compounds. It would seem at first pass that a ring substitution on the 2-benzyl is key for heightened conformation at the 5ht2a receptor.
ebola
the identity and purity of this compound is open to question. I would begin with lower doses than these (in the range of NBOMe substituted 2Cs) until more reports flow in.
...
It would be really interesting if this were to pan out though, as it would begin to map the SAR of this class of compounds. It would seem at first pass that a ring substitution on the 2-benzyl is key for heightened conformation at the 5ht2a receptor.
ebola
amanitadine
Bluelighter
Everything I have seen seems to point that the bulky oxygen is needed there for the increased efficacy, whether it be a MeO, MDO, or HO group. I am kinda surprised that N-Benzyl 2C-B is active at all, considering how other N-Benzyl PEAS have panned out...