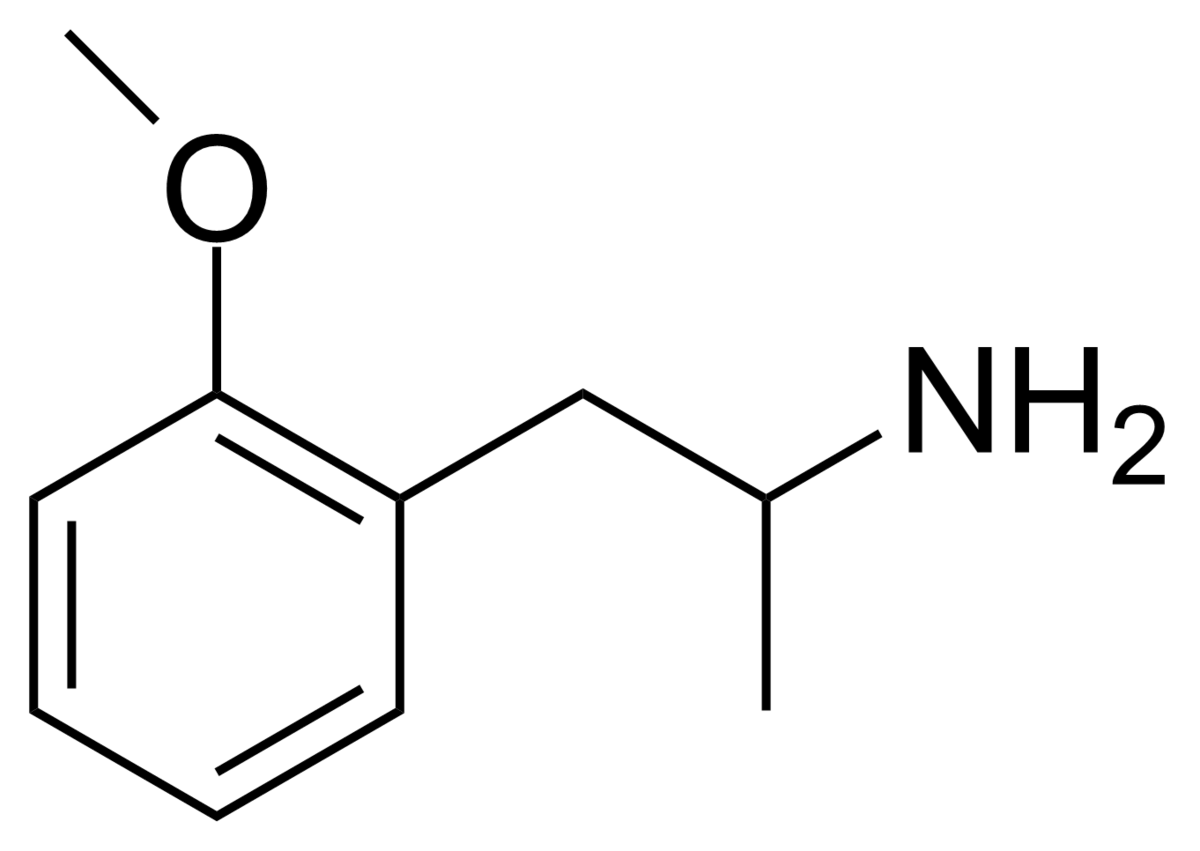
Hello guys, just wanted to give a quick update:
I got my order about a day after I made my last post here, and since then have done 8 doses (anywhere from 15-25mg), oral, at the start of the day. Apart from the first 2 doses that were done on consecutive days, the other 6 doses were spread out.
I weighed my doses using a milligram scale, that has been calibrated beforehand, then placing a 10g weight and then adding my desired dose carefully.
Apart from 2-FMA, I also have a cup or two of coffee a day almost daily, with my morning coffee taken in conjunction with my powder, and then another refill mid-day.
Things I've noticed:
- An increase in my desire to socialise (+)
- Willingness to perform tedious tasks (+)
- Anxiety reduction (+)
- Tightness and soreness in my chest area (-)
- Racing thoughts (-)
From my short time with the compound, I have been quite impressed with its performance. It did its job and more as a functional stimulant, I found myself being much more task-oriented, and enjoyed socialising with new people, all whilst sober.
I had days where I had to present my work to my lab, and where I would normally be anxious, I didn't get the normal butterflies or nervous energy, but rather a subtle yet self-assured aura that allowed me to articulate myself better. My prep wasn't anything different, I just walked in with a clear goal of presenting my work, and wasn't clouded with any of the anxiety or self-doubt.
However, I did note that throughout the days I did take 2-FMA, I felt this dulling sensation in my chest area, that also manifested into soreness. This wasn't anything uncomfortable, but was definitely there.
Also, most mornings, I would go for a morning jog after my coffee, and I've noticed my heart beat was a lot more erratic, which is weird as I always pace myself and take it gentle.
I thought maybe the coffee could have contributed to this, so after a 2 day break, I took about 20mg and did another morning jog, and my heart beat was noticeably more calm and smooth.
If I had to compare it to Modafinil, I'd say 2-FMA is similar in the sense that most of the effects are quite subtle and in-the-background, I didn't notice any physical sensations, euphoria, or forced actions. I didn't suddenly gain a massive bout of motivation to do XYZ, but starting tasks was a lot more easier. I don't have an addictive personality, so I've also never felt the urge to redose.
Going forward, I won't have a fixed dosing protocol, I'll be taking it sporadically whenever I have a busy day ahead, and never take it on consecutive days. The dull sensation I felt, whilst nothing uncomfortable, is still something that is on the back of my mind, so I'll be quite mindful and monitor my body for any warning signs.
I've planned on documenting my process as I use 2-FMA, so I'll probably be posting semi-frequent updates as I become more experienced with this compound. Hopefully new people reading this who are thinking of starting 2-FMA can find something useful from my reports. 2-FMA is definitely not a magic drug, you need to put your best foot forward to utilise this to its full capabilities, and your body is different to mine, what I'm currently experiencing during this honey moon phase may not be the same for you.
Cheers, and stay safe everyone
I got my order about a day after I made my last post here, and since then have done 8 doses (anywhere from 15-25mg), oral, at the start of the day. Apart from the first 2 doses that were done on consecutive days, the other 6 doses were spread out.
I weighed my doses using a milligram scale, that has been calibrated beforehand, then placing a 10g weight and then adding my desired dose carefully.
Apart from 2-FMA, I also have a cup or two of coffee a day almost daily, with my morning coffee taken in conjunction with my powder, and then another refill mid-day.
Things I've noticed:
- An increase in my desire to socialise (+)
- Willingness to perform tedious tasks (+)
- Anxiety reduction (+)
- Tightness and soreness in my chest area (-)
- Racing thoughts (-)
From my short time with the compound, I have been quite impressed with its performance. It did its job and more as a functional stimulant, I found myself being much more task-oriented, and enjoyed socialising with new people, all whilst sober.
I had days where I had to present my work to my lab, and where I would normally be anxious, I didn't get the normal butterflies or nervous energy, but rather a subtle yet self-assured aura that allowed me to articulate myself better. My prep wasn't anything different, I just walked in with a clear goal of presenting my work, and wasn't clouded with any of the anxiety or self-doubt.
However, I did note that throughout the days I did take 2-FMA, I felt this dulling sensation in my chest area, that also manifested into soreness. This wasn't anything uncomfortable, but was definitely there.
Also, most mornings, I would go for a morning jog after my coffee, and I've noticed my heart beat was a lot more erratic, which is weird as I always pace myself and take it gentle.
I thought maybe the coffee could have contributed to this, so after a 2 day break, I took about 20mg and did another morning jog, and my heart beat was noticeably more calm and smooth.
If I had to compare it to Modafinil, I'd say 2-FMA is similar in the sense that most of the effects are quite subtle and in-the-background, I didn't notice any physical sensations, euphoria, or forced actions. I didn't suddenly gain a massive bout of motivation to do XYZ, but starting tasks was a lot more easier. I don't have an addictive personality, so I've also never felt the urge to redose.
Going forward, I won't have a fixed dosing protocol, I'll be taking it sporadically whenever I have a busy day ahead, and never take it on consecutive days. The dull sensation I felt, whilst nothing uncomfortable, is still something that is on the back of my mind, so I'll be quite mindful and monitor my body for any warning signs.
I've planned on documenting my process as I use 2-FMA, so I'll probably be posting semi-frequent updates as I become more experienced with this compound. Hopefully new people reading this who are thinking of starting 2-FMA can find something useful from my reports. 2-FMA is definitely not a magic drug, you need to put your best foot forward to utilise this to its full capabilities, and your body is different to mine, what I'm currently experiencing during this honey moon phase may not be the same for you.
Cheers, and stay safe everyone
Last edited: