Synaps3
Bluelighter
- Joined
- Sep 14, 2011
- Messages
- 257
I used to use 2m2b a lot several years ago, but now I use ECX only and not that often (once a week max). I take kratom daily (it's the only drug I take daily). The ECX is known to deplete enzymes, but I found it to not have a perceptible effect on that.
Anyway, for someone that is well versed in pharmacology, how long do you think this stuff stays in the body? I can feel the effect (although mild - about 18 hours after). I suspect it is just getting distributed into the fat tissue causing the effects to seem to go away, but I suspect it stays in the body for longer. Does anyone have an idea based on the structure? I'm just thinking that having that triple bond in the body for a very long time probably isn't good cause I believe it is pretty reactive (correct if I'm wrong).
The half-life is only 2.5 hours, but it doesn't actually get removed from the body in that time. I wonder what is the term used for half-life of body elimination?
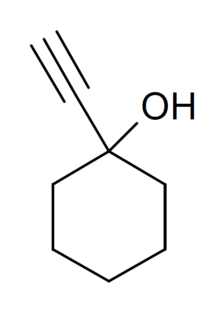
Anyway, for someone that is well versed in pharmacology, how long do you think this stuff stays in the body? I can feel the effect (although mild - about 18 hours after). I suspect it is just getting distributed into the fat tissue causing the effects to seem to go away, but I suspect it stays in the body for longer. Does anyone have an idea based on the structure? I'm just thinking that having that triple bond in the body for a very long time probably isn't good cause I believe it is pretty reactive (correct if I'm wrong).
The half-life is only 2.5 hours, but it doesn't actually get removed from the body in that time. I wonder what is the term used for half-life of body elimination?

