-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
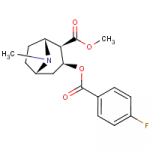
4 Fluoro-Cocaine
- Thread starter jackdaw
- Start date
Nagelfar
Bluelight Crew
Cocaine stimulant homologues and analogues interest me very much, but there is so little information out there! I always wondered how a covalent binding cocaine homologue would be different from the phosphoryating effect of amphetamine in duration. Longer or shorter? More euphoric or less euphoric? I have the feeling, due to it's keeping the monoamine in question in it's basic functional form, that it would be a smoother, more euphoric, feeling. Just for a longer duration of time (but am unsure whether it would be longer than, for example, methamp).
As for 4 fluoro-cocaine in particular, I have no information I'm sorry.
As for 4 fluoro-cocaine in particular, I have no information I'm sorry.
Last edited:
Some information I dug up.
It doesn't neccessarily answer all my questions, but after some digging, this is what I found;
"I've heard that cocaine with a 4-fluoro on it is about the same but 60 times stronger. It is also uncontrolled in Canada, as in fact are all cocaine analogs...
I have seen the synthesis of the compound before, but I have not found an actual journal about the effects (one site had a name and a date, not further reference to something someone could actually find). So I can't really say if the above is true but it seems to be decently well known on various clandestine chemistry sites. It's just nobody has the reference!
I also saw an analog that had some extremely high potencies for blocking dopamine reuptake, it replaced the normal methyl ester on the cocaine molecule with a plain propyl group (no ester) and a 4-fluoro aromatic ring also directly bonded to the central tropine ring.
It seems that in any case, 4-fluoro rings are always more potent than the plain compounds.
Now, here is a question- wouldn't blocking dopamine re-uptake always make the user feel great? A lot of analogs have very high strengths for causing dopamine levels to shoot through the roof. However, many don't seem to replace cocaine very well, according to the monkeys who get tested all the time. Couldn't it just be that they are not triggering some other receptor involved in cocaine addiction, and that the drugs themselves ARE abusable but simply don't replace cocaine because they aren't mimicking all of it's effects?
It could be that all of these cocaine analogs that don't replace it for addicts might have great possibilities as recreational compounds, it's just no human ever bothered to snort his research project to see how good it was, and when the monkies wanted their crack back they just decided it had no potential as a recreational compound.
On 4-fluorococaine I managed to locate the following paper:
Journal of Neurochemistry Volume 62 Page 1154 (March 1994)
http://rapidshare.de/files/32780562/4-fluorococaine.pdf.html
from page 7:
"There is evidence that inhibition of DA
reuptake by cocaine is responsible for producing euphoria
and its consequent abuse (Ritz et al., 1987).
The PET results with labeled 4'-fluorococaine imply
that para substitution with fluorine does not alter cocaine's
interaction with its binding sites in the brain.
The in vitro competition experiments supported this
implication, because cocaine and 4'-fluorococaine
had similar affinities for the DA reuptake site. They
also exhibited similar affinities for NE reuptake sites.
However, 4'-fluorococaine was about 100 times more
potent than cocaine at the rat brainstem 5-HT reuptake
site labeled with [3H]paroxetine . This observation,
together with the nearly identical striatal kinetics
oflabeled cocaine and 4'-fluorococaine, confirms that
binding to 5-HT reuptake sites is not important in the
striatal uptake of ["C]cocaine. No increased uptake
of labeled 4'-fluorococaine relative to cocaine was
seen in any regions, including the brainstem and frontal
cortex . Presumably, the small size of the baboon
raphe nuclei (1-2 mm) precluded visualization in a
tomograph with spatial resolution limited to 6 mm.
Frontal cortex contains 5-HT reuptake sites, but their
concentration is lower than that of DA reuptake sites
in the striatum . The affinity of 4'-fluorococaine for
5-HT reuptake sites may be too low, or the dissociation
rate constant too rapid, for visualization of frontal
cortex 5-HT reuptake sites against the background
of nonspecifically bound radiotracer. Our ICSO value
of 7.5 nM (Table 3) corresponds (Cheng and Prusofh
1973) to a K; for 4'-fluorococaine for the 5-HT transporter
of 0.3 nM assuming a KD of20 pM for paroxetine
(Ritz et al., 1990). Tritiated paroxetine does preferentially
label in vivo brain regions relatively rich in
5-HT transporters (Scheffel and Hartig, 1989), perhaps
because its affinity is 15-fold higher than that of
4'-fluorococaine . It also binds to nonserotonergic
sites, however (Biegon and Mathis, 1993)."
(My apologies for the Rosco Bodine-quality of formatting)
In my point of view these tropacocaine analogs seem interesting curiosities. Although not as potent as cocaine, they are (relatively) easier synthesised than cocaine analogs and they are legal here in the Carolingian Empire (cocaine analogs too).
Studies with differentially labeled [11C]cocaine,[11C]norcocaine,[11C]benzoylecgonine, and [11C]- and 4'-[18F]fluorococaine to probe the extent to which [11C]cocaine metabolites contribute to PET images of the baboon brain.
[My paper] S J Gatley, D W Yu, J S Fowler, R R MacGregor, D J Schlyer, S L Dewey, A P Wolf, T Martin, C E Shea, N D Volkow Department of Chemistry, Brookhaven National Laboratory, Upton, New York 11973. The psychostimulant drug of abuse, cocaine (benzoylecgonine methyl ester), is rapidly metabolized by cleavage of its two ester groups, to give benzoylecgonine (BE) and ecgonine methyl ester, and by N-demethylation, to give N-norcocaine (NC). The recent use of [N-methyl-11CH3]cocaine to image brain cocaine binding sites with positron emission tomography (PET) raises the question of whether PET images partially reflect the distribution and kinetics of labeled cocaine metabolites. We prepared [O-methyl-11CH3]cocaine by methylation of the sodium salt of BE with [11C]CH3I, and showed that PET baboon brain scans, as well as regional brain kinetics and plasma time-activity curves corrected for the presence of labeled metabolites, are nearly identical to those seen with [N-methyl-11CH3]cocaine. This strongly suggests that 11C metabolites do not significantly affect PET images, because the metabolite pattern is different for the two labeled forms of cocaine. In particular, nearly half the 11C in blood plasma at 30 min was [11C]CO2 when [N-methyl-11CH3]cocaine was administered, whereas [11C]CO2 was not formed from [O-methyl-11CH3]cocaine. Only a trace of [11C]NC was detected in plasma after [O-methyl-11CH3]cocaine administration. Nearly identical brain PET data were also obtained when 4'-[N-methyl-11CH3]fluorococaine and 4'-[18F]fluorococaine (prepared by nucleophilic aromatic substitution from [18F]fluoride- and 4'-nitrococaine) were compared with [N-methyl-11CH3]cocaine. In vitro assays with rat brain membranes showed that cocaine and 4'-fluorococaine were equipotent at the dopamine reuptake site, but that 4'-fluorococaine was about 100 times more potent at the 5-hydroxytryptamine reuptake site.(ABSTRACT TRUNCATED AT 250 WORDS) "
It doesn't neccessarily answer all my questions, but after some digging, this is what I found;
"I've heard that cocaine with a 4-fluoro on it is about the same but 60 times stronger. It is also uncontrolled in Canada, as in fact are all cocaine analogs...
I have seen the synthesis of the compound before, but I have not found an actual journal about the effects (one site had a name and a date, not further reference to something someone could actually find). So I can't really say if the above is true but it seems to be decently well known on various clandestine chemistry sites. It's just nobody has the reference!
I also saw an analog that had some extremely high potencies for blocking dopamine reuptake, it replaced the normal methyl ester on the cocaine molecule with a plain propyl group (no ester) and a 4-fluoro aromatic ring also directly bonded to the central tropine ring.
It seems that in any case, 4-fluoro rings are always more potent than the plain compounds.
Now, here is a question- wouldn't blocking dopamine re-uptake always make the user feel great? A lot of analogs have very high strengths for causing dopamine levels to shoot through the roof. However, many don't seem to replace cocaine very well, according to the monkeys who get tested all the time. Couldn't it just be that they are not triggering some other receptor involved in cocaine addiction, and that the drugs themselves ARE abusable but simply don't replace cocaine because they aren't mimicking all of it's effects?
It could be that all of these cocaine analogs that don't replace it for addicts might have great possibilities as recreational compounds, it's just no human ever bothered to snort his research project to see how good it was, and when the monkies wanted their crack back they just decided it had no potential as a recreational compound.
On 4-fluorococaine I managed to locate the following paper:
Journal of Neurochemistry Volume 62 Page 1154 (March 1994)
http://rapidshare.de/files/32780562/4-fluorococaine.pdf.html
from page 7:
"There is evidence that inhibition of DA
reuptake by cocaine is responsible for producing euphoria
and its consequent abuse (Ritz et al., 1987).
The PET results with labeled 4'-fluorococaine imply
that para substitution with fluorine does not alter cocaine's
interaction with its binding sites in the brain.
The in vitro competition experiments supported this
implication, because cocaine and 4'-fluorococaine
had similar affinities for the DA reuptake site. They
also exhibited similar affinities for NE reuptake sites.
However, 4'-fluorococaine was about 100 times more
potent than cocaine at the rat brainstem 5-HT reuptake
site labeled with [3H]paroxetine . This observation,
together with the nearly identical striatal kinetics
oflabeled cocaine and 4'-fluorococaine, confirms that
binding to 5-HT reuptake sites is not important in the
striatal uptake of ["C]cocaine. No increased uptake
of labeled 4'-fluorococaine relative to cocaine was
seen in any regions, including the brainstem and frontal
cortex . Presumably, the small size of the baboon
raphe nuclei (1-2 mm) precluded visualization in a
tomograph with spatial resolution limited to 6 mm.
Frontal cortex contains 5-HT reuptake sites, but their
concentration is lower than that of DA reuptake sites
in the striatum . The affinity of 4'-fluorococaine for
5-HT reuptake sites may be too low, or the dissociation
rate constant too rapid, for visualization of frontal
cortex 5-HT reuptake sites against the background
of nonspecifically bound radiotracer. Our ICSO value
of 7.5 nM (Table 3) corresponds (Cheng and Prusofh
1973) to a K; for 4'-fluorococaine for the 5-HT transporter
of 0.3 nM assuming a KD of20 pM for paroxetine
(Ritz et al., 1990). Tritiated paroxetine does preferentially
label in vivo brain regions relatively rich in
5-HT transporters (Scheffel and Hartig, 1989), perhaps
because its affinity is 15-fold higher than that of
4'-fluorococaine . It also binds to nonserotonergic
sites, however (Biegon and Mathis, 1993)."
(My apologies for the Rosco Bodine-quality of formatting)
In my point of view these tropacocaine analogs seem interesting curiosities. Although not as potent as cocaine, they are (relatively) easier synthesised than cocaine analogs and they are legal here in the Carolingian Empire (cocaine analogs too).
Studies with differentially labeled [11C]cocaine,[11C]norcocaine,[11C]benzoylecgonine, and [11C]- and 4'-[18F]fluorococaine to probe the extent to which [11C]cocaine metabolites contribute to PET images of the baboon brain.
[My paper] S J Gatley, D W Yu, J S Fowler, R R MacGregor, D J Schlyer, S L Dewey, A P Wolf, T Martin, C E Shea, N D Volkow Department of Chemistry, Brookhaven National Laboratory, Upton, New York 11973. The psychostimulant drug of abuse, cocaine (benzoylecgonine methyl ester), is rapidly metabolized by cleavage of its two ester groups, to give benzoylecgonine (BE) and ecgonine methyl ester, and by N-demethylation, to give N-norcocaine (NC). The recent use of [N-methyl-11CH3]cocaine to image brain cocaine binding sites with positron emission tomography (PET) raises the question of whether PET images partially reflect the distribution and kinetics of labeled cocaine metabolites. We prepared [O-methyl-11CH3]cocaine by methylation of the sodium salt of BE with [11C]CH3I, and showed that PET baboon brain scans, as well as regional brain kinetics and plasma time-activity curves corrected for the presence of labeled metabolites, are nearly identical to those seen with [N-methyl-11CH3]cocaine. This strongly suggests that 11C metabolites do not significantly affect PET images, because the metabolite pattern is different for the two labeled forms of cocaine. In particular, nearly half the 11C in blood plasma at 30 min was [11C]CO2 when [N-methyl-11CH3]cocaine was administered, whereas [11C]CO2 was not formed from [O-methyl-11CH3]cocaine. Only a trace of [11C]NC was detected in plasma after [O-methyl-11CH3]cocaine administration. Nearly identical brain PET data were also obtained when 4'-[N-methyl-11CH3]fluorococaine and 4'-[18F]fluorococaine (prepared by nucleophilic aromatic substitution from [18F]fluoride- and 4'-nitrococaine) were compared with [N-methyl-11CH3]cocaine. In vitro assays with rat brain membranes showed that cocaine and 4'-fluorococaine were equipotent at the dopamine reuptake site, but that 4'-fluorococaine was about 100 times more potent at the 5-hydroxytryptamine reuptake site.(ABSTRACT TRUNCATED AT 250 WORDS) "
I always wondered how a covalent binding cocaine homologue would different from the phosphoryating effect of amphetamine in duration.
You speak chemistry well
Nagelfar
Bluelight Crew
Potency to activate/bind-to the rewarding receptor associated with the drug doesn't equal the euphoria it does induce. Otherwise people would choose fentanyl over heroin or oxycodone. However, the area of conformation and what part of the receptor it bonds to, rather than how "tightly" or well it bonds, seems to be the reason for the euphoria. See my thread here and the interpretation of the data presented:
http://www.bluelight.ru/vb/showthread.php?p=6711122
http://www.bluelight.ru/vb/showthread.php?p=6711122
i wonder if this will make it onto the streets as coke and is it the same dosage for people that would inject this stuff?
cos it looks like ur just wondering imagine the overdose's
coke users beware.
what effect does that para group have on coke i wonder same effects as amphetamines i hope not.
toxic for the heart i wonder if it more toxic or not.
hmmm i wish i had some coke
cos it looks like ur just wondering imagine the overdose's
coke users beware.
what effect does that para group have on coke i wonder same effects as amphetamines i hope not.
toxic for the heart i wonder if it more toxic or not.
hmmm i wish i had some coke
phase_dancer
Bluelight Crew
jackdaw, your rapidshare link is dead
Like the SM sense of humour
Like the SM sense of humour
Hey phase_dancer, what's rapidshare?
Forgive me I'm a bit ignorant and coming down with a vengeance from a night tasting methylmethcat/mephedrone. I'm pretty wrecked, though not overly impressed. Many report MDMA like effects. It's years since I did a pill, they must be really shit, these days if there like mephedrone. I'd describe more amphetamine sulphate with the jaw clenching and without the forced euphoria. I'm unlikely to repeat after I finish the last 200 mg's.
I need to lie down and close my eyes it's nearly nine in the morning here. I think I've made about 50-60 posts last night. Jeezus I never thought I could type that much - friggin stimulants..lol
Forgive me I'm a bit ignorant and coming down with a vengeance from a night tasting methylmethcat/mephedrone. I'm pretty wrecked, though not overly impressed. Many report MDMA like effects. It's years since I did a pill, they must be really shit, these days if there like mephedrone. I'd describe more amphetamine sulphate with the jaw clenching and without the forced euphoria. I'm unlikely to repeat after I finish the last 200 mg's.
I need to lie down and close my eyes it's nearly nine in the morning here. I think I've made about 50-60 posts last night. Jeezus I never thought I could type that much - friggin stimulants..lol

Tadfish- I'm possibly coming into possesion of a small amount, in the next few weeks. The dosage seems to be a matter of debate and/or opinion, but appears to be between 60 -100 times the potency of cocaine hcl.
I'm very wary about introducing this stuff to my CNS. I'm not a fan of stimulants, in particular the more potent ones. I find myself losing my hard one self control with them and end up in a mess. The sample of 4fluorococaine will probably be stashed away until I can get more information. I've searched everywhere I know of on the net and there's very little knowledge to be had.
Hoping, there's someone else out there who can add to this thread at some point.
I'm very wary about introducing this stuff to my CNS. I'm not a fan of stimulants, in particular the more potent ones. I find myself losing my hard one self control with them and end up in a mess. The sample of 4fluorococaine will probably be stashed away until I can get more information. I've searched everywhere I know of on the net and there's very little knowledge to be had.
Hoping, there's someone else out there who can add to this thread at some point.
morphene
Bluelighter
- Joined
- Mar 22, 2009
- Messages
- 543
i wonder if this will make it onto the streets as coke
I'm looking at a new list with 4-FC on it for pretty damn cheap.
And then directly below it, pretty out of order is:
Novocaine Hcl(procaine) crystals pharma grade, one eur per gram
Now I don't do coke, but uh, I'm thinking that is pretty easy for anyone to put together that reads the list, heh.
So, no one here ended up trying it? I'm tempted just because it's novel and inexpensive, but cocaine barely does anything to me, just makes me chatty and makes me fiend. So I'm probably the worst possible test subject to see if it does anything.
I hope to try some out soon going traveling. but is it legal and if so where? are there cocaine analog laws? i wonder 60times stronger imagine that shit hitting the streets!
of course it is not legal in many places, in the US it is an analogue of cocaine therefore illegal, in the UK it is illegal class A as are all similar modifications of cocaine.
Tadfish- I'm possibly coming into possesion of a small amount, in the next few weeks. The dosage seems to be a matter of debate and/or opinion, but appears to be between 60 -100 times the potency of cocaine hcl.
I'm very wary about introducing this stuff to my CNS. I'm not a fan of stimulants, in particular the more potent ones. I find myself losing my hard one self control with them and end up in a mess. The sample of 4fluorococaine will probably be stashed away until I can get more information. I've searched everywhere I know of on the net and there's very little knowledge to be had.
Hoping, there's someone else out there who can add to this thread at some point.
Did you get any, and if so, have you tried it?
Hammilton
Bluelighter
- Joined
- Sep 2, 2008
- Messages
- 3,435
... no.
Where cocaine synthesis would start with the appropriately unsubstituted precursor, para-fluorococaine would start with the para-fluorinated cocaine derivative. I assume, anyway. It would have to start with something else on that phenyl ring though. Pretty sure there's no way to add a fluorine atom to a plain ring like that.
Where cocaine synthesis would start with the appropriately unsubstituted precursor, para-fluorococaine would start with the para-fluorinated cocaine derivative. I assume, anyway. It would have to start with something else on that phenyl ring though. Pretty sure there's no way to add a fluorine atom to a plain ring like that.
Hammilton
Bluelighter
- Joined
- Sep 2, 2008
- Messages
- 3,435
Ah, right. 4-FC makes more sense than 4-FTC. If Wikipedia is right, and I haven't read the reference cited yet, 4-FTC has only 30% the stimulant activity, but roughly equal potency as a local aneasthetic.
That would make dimethocaine a safer alternative- though info on any hepatotoxicity would be nice.
That would make dimethocaine a safer alternative- though info on any hepatotoxicity would be nice.
heard at least one batch is not stimulating at all with anti cholinagenic effects even sedating.
hmmmm.
Is the difference between that tropicocaine or whatever its called mybe my friend got this.
I am interested cause as downers like opiates get more potent and potent with like opiates 10,000 times stronger than morphine and stuff.
imagine if improvements on stims went that way as well.
thats what get me excited about 4-fluro-coke.
think everyone one would love it cause coke is so impure in australia and it would be mad to get really strong coke effects without snorting heaps and impurities.
Wish i was in Asian trying some now. Interesting if it has to be made from illegal starting chemicals cocaine. Wonder if its cheap to make and then the world might see more of it. as i cheap way of making 60 times more out of ya coke profit.
hmmmm.
Is the difference between that tropicocaine or whatever its called mybe my friend got this.
I am interested cause as downers like opiates get more potent and potent with like opiates 10,000 times stronger than morphine and stuff.
imagine if improvements on stims went that way as well.
thats what get me excited about 4-fluro-coke.
think everyone one would love it cause coke is so impure in australia and it would be mad to get really strong coke effects without snorting heaps and impurities.
Wish i was in Asian trying some now. Interesting if it has to be made from illegal starting chemicals cocaine. Wonder if its cheap to make and then the world might see more of it. as i cheap way of making 60 times more out of ya coke profit.