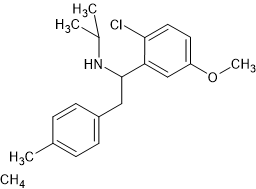
Reports of tiletamine itself are somewhat mixed. It's interesting to note that the QSAR of this class is sufficiently understood to make predictions. It is not clear if ring-substitution of the thiophene ring is worthwhile. It could be argued that the S of the thiophene ring should be counted as a substitution. While Reaxys listed a paper in which a furan ring was the aromatic, the paper could not be found.
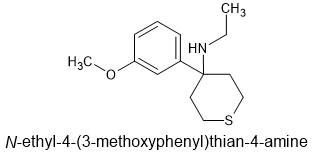
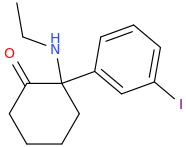
I think it's time to move from the cyclohexyl moiety. It's presence is biosteric and the 4-thianes have been investigated and shown to be MORE active. That is, affinity increases. It would seem obvious to make the 4-thiane homologue of MXE and then 2-chloro MXE & then their N-methyl homologues. Quite a few compounds.
Of course, it's the fact that the (S) enantiomers are NMDA antagonists & the (R) enantiomers are DRIs that is crucial to subjective effects. It's very simply to produce pure DRI & pure NMDA antagonists by resolving the isomers or reading the QSAR of the classes to suppress one of the actions.