Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627

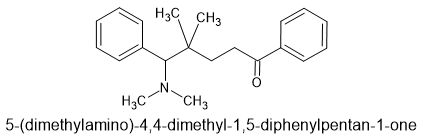
As strong as demerol, 1 step from 2 common precursors.
N&PD Moderators: Skorpio | someguyontheinternet

The laymen that post here aren't going to understand any of this. You and fastandbulbous do this in Neuro.
Going to move the thread.
I'm sure you know by reading the first 4 pages of this sub forum , and the questions that are asked, this thread is way beyond their comprehension.
Didn't say it had to do with synthesis. The thread won't be comprehended in BDD and I think you know that.Well fine - but it isn't to do with synthesis - it's purely a simple pattern-matching. But as you see fit.
Didn't say it had to do with synthesis. The thread won't be comprehended in BDD and I think you know that.
I get it. I hear ya. You like to post a lot. That's fine. I know you are educating people. But threads belong in certain sub forums so just go with the flow.People can ask - I LIKE people to ask. That's how education works. I've posted 1001 novel compounds and in 99% of cases, people ask, get an explaination and move on. The few who still struggle, I go to greater depths with reference.
It's all over the site - I don't do it as a one-off. Once people know of Chemdraw, Pubchem, RO5 and other free assets, it's not actually hard. OK we get the 1% who glory in not using assets and claiming it as some kind of victory - but those people tend to resort to swearing.... as they did yesterrday.

im in the process of working with some post-docs on small molecules and getting them to have higher binding affinity, well I am producing and making while they are doing the real research and telling me what reactions to do etcNote similarity to:

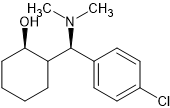
Although the latter has a chiral amine and no paper resolves them.
This work is also covered by CA Patent 830475A 'Analgesic cyclobutanes derivative compositions'
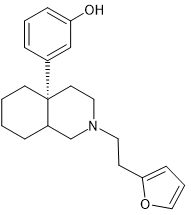
What is interesting is that this class is similar to Lednicer's (much more potent) BDPC (and homologues). The above has more rotatable bonds and is not the ideal biosteric minimum. I have no idea if adding a p-Br to the benzylamine would increase affinity, but it seems quite likely. With this class, if one can obtain the precursor, it is synthetically simple.
There are many workers who had limited funding for the research of opioids. Until the 1970s nobody really understood what requirements a mu agonist required, but now there are papers with training-sets & 3D QSAR.
You in a med chem program? I'm more in the life sciences arena and often romanticize organic chemists, sometimes I wish I went that route (though I love having shirts without acid holes in them).im in the process of working with some post-docs on small molecules and getting them to have higher binding affinity, well I am producing and making while they are doing the real research and telling me what reactions to do etc