-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989
No, but Siriusly:
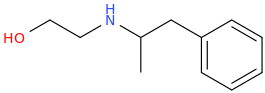
SYDNEY
2-(N-hydroxyethylamino)-1-phenylpropane
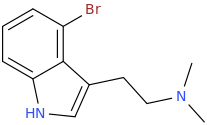
PERTH
1-(4-bromoindole-3-yl)-2-dimethylaminoethane
The West (oeste) Is Associated With Wisdom; The East (este) With Money, Property, & Prestige.
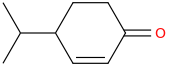
LAVENDAR
1-isopropyl-4-oxocyclohex-2-ene

SYDNEY
2-(N-hydroxyethylamino)-1-phenylpropane

PERTH
1-(4-bromoindole-3-yl)-2-dimethylaminoethane
The West (oeste) Is Associated With Wisdom; The East (este) With Money, Property, & Prestige.

LAVENDAR
1-isopropyl-4-oxocyclohex-2-ene
Last edited:
Jabberwocky
Frumious Bandersnatch
Does that mean Olanzapine was approved just a little while ago? i been prescribed it for ages yet i never took it because it puts me to sleep and it does something to serotonin i think it blocks it and that would not be any good for me
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989
Zyprexa's been out since the mid-1990's.
I'm actually thinking about trying this new 2 in 1 antipsychotic later this year when it comes out. The reason being that I am curious about what's so great about being addicted to opiates. Yes, I know that 'curiousity killed the cat." But, like Schrodinger's cat, this here cat is BOTH alive & dead! And loved either way.
I'm actually thinking about trying this new 2 in 1 antipsychotic later this year when it comes out. The reason being that I am curious about what's so great about being addicted to opiates. Yes, I know that 'curiousity killed the cat." But, like Schrodinger's cat, this here cat is BOTH alive & dead! And loved either way.

Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989

JONES 21
1-(1,3-benzodioxole-5-yl)-2-(2-hydroxyethylamino)propane

PENNINGTON 48
2-methylamino-1-(4-amylphenyl)-propane
Sister Aimee
The Torah
The Talmud
The Pentateuche
The Koran
The Tao te Ching by Lao Tzu
The Religions of Man by Huston Smith
The Bhaghavad Gita
The Rig Vedas
Suzanne Vega
Viva Las Vegas, 'the fertile (!) plane.'
VISA Green Cards R.E.M. Madonna. Ciccone Jennifer Cohen
J.P. Morgan Chase The Muppets Took The Manhattan Project
Going Viral Is A Thing.
Linkin Park.
A Pill Press.
North Druid Hills.
"I Will Lift Up My Hands Unto The Hills From Whence My Salvation Comes."--La Santa Biblia. Radiohead. Augusta, ME (Maine). Same Name Fun & Games. A Digital Eternity.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989

PLURIPOTENT
1-(4-methoxyphenyl)-1,3-bis-(dichloroacetoxy)-2-(dichloroacetamido)propane
-investigational new antibiotic / wart, skin cancer, age spot chemical cauterizer
-inspired by chloramphenicol, a natural product antibiotic found in the ocean
EDIT: When In Doubt, Leave The Stereochemistry Out.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989
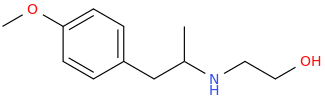
RASPBERRY_CHAMPAGNE.
1-(4-methoxyphenyl)-2-(2-hydroxyethylamino)propane
Cleverness Hace Lo Que Se Puede.
¡Genius Hace Lo Que Ich MuB!
We Are Living In The Land Of Shadows.
Take A Walk Through The Crystal Meadows.
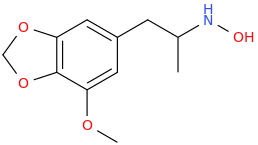
SID_CAESAR_AUGUSTUS.
1-(3,4-methylenedioxy-5-methoxyphenyl)-2-hydroxylaminopropane
Pizza! Pizza!
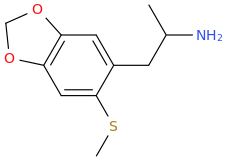
ZUESY_KIM.
1-(3,4-methylenedioxy-6-(methylthio)phenyl)-2-aminopropane
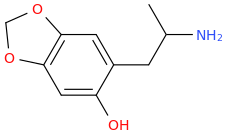
OPEN_SESAME_STREET'S_BEATIFIC_BEAT.
1-(3,4-methylenedioxy-6-hydroxyphenyl)-2-aminopropane
Las Puertas Estan Abiertas.
Yo Soy La Llave.
Last edited:
Jabberwocky
Frumious Bandersnatch
bruhhhZyprexa's been out since the mid-1990's.
I'm actually thinking about trying this new 2 in 1 antipsychotic later this year when it comes out. The reason being that I am curious about what's so great about being addicted to opiates. Yes, I know that 'curiousity killed the cat." But, like Schrodinger's cat, this here cat is BOTH alive & dead! And loved either way.
i wish you luck with that
as in, i hope you dont have to get addicted cuz its hell you kinda stop giving a fuck about things that once made u happy until opiates are your only happiness
Truth.bruhhh
i wish you luck with that
as in, i hope you dont have to get addicted cuz its hell you kinda stop giving a fuck about things that once made u happy until opiates are your only happiness
Any addiction sucks, but opiate addiction REALLY really sucks.
izo
Bluelighter
Synthesis and analgesic properties of N-substituted trans-4a-aryldecahydroisoquinolines
Abstract
A representative series of N-substituted derivatives of the morphine-based trans-4a-aryldecahydroisoquinoline were synthesized and evaluated for opioid analgesic activities. Compounds with potent analgesic activity and high affinities for the mu and kappa opioid receptors were discovered. The effect of varying the N-substituent in the trans-4a-aryldecahydroisoquinoline paralleled, to a certain extent, previous findings with other morphine part structures. Replacement of the N-methyl with a phenethyl group significantly increased analgesic potency. The N-cyclopropylmethyl analogue was found in rodents to have mixed agonist-antagonist properties; however, its antagonist activity was far weaker than those reported for the N-(cyclopropylmethyl)morphinan and -benzomorphan derivatives. Resolution of the stereoisomers and determination of their absolute configuration by X-ray crystallography showed that the opioid receptor effects were predominantly found with the 4aR,8aR isomer, the same relative absolute configuration of morphine. Unexpectedly, the 4aR,8aR N-cyclopropylmethyl analogue (compound 30), which in rodents had mixed agonist-antagonist properties similar to those of pentazocine, was found in rhesus monkeys to behave as a full morphine-like agonist.

Synthesis and analgesic properties of N-substituted trans-4a-aryldecahydroisoquinolines - PubMed
A representative series of N-substituted derivatives of the morphine-based trans-4a-aryldecahydroisoquinoline were synthesized and evaluated for opioid analgesic activities. Compounds with potent analgesic activity and high affinities for the mu and kappa opioid receptors were discovered. The...
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989

WIDGET
1-(2-hydroxyethyl)piperidin-4-yl
phenyl ether

FIDGET
1-(2-hydroxyethyl)piperidin-4-yl 3,4-methylenedioxyphenyl ether
NaOMe (Na+[-O-CH3])
NAOMI
Sodium Methoxide
The Williamson Ether Synthesis

CHRIS
3,5-dimethoxy-1-(2-aminoethyl)-benzene-4-yl (2-hydroxyethyl)piperidine-4-yl ether

RICK
2,6-dimethoxy-1-(2-aminoethyl)-benzene-4-yl-(2-aminopropane)

LIL DANNY
1-(4-ethylphenyl)-2-aminopropane
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 6,989

PEEWEE
N-isopropyl-1-phenyl-2-aminoethane

ANDOUILLE
1-phenyl-2-(dichloroacetamido)propane

GUBERNATORIAL
1-(4-ethoxy-3-methoxyphenyl)-2-methylaminopropan-1-one

HYBRID_THEORY
N-(1-methyl-2-phenylethyl)-2-(indole-3-yl)ethyl-N-methylamine

AWAKE
N-(1-methyl-2-phenylethyl)-2-(indole-3-yl)-(1-methylethyl)amine
Sometimes I Take Medicine To Kill Neurons. But Usually I Take Medicines To Regrow Neurons.

ELASTIC_ELATEMENT
1-(4-hydroxyindole-3-yl)-2-aminopropane
Last edited:
izo
Bluelighter
Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug
Abstract
Phenibut (beta-phenyl-gamma-aminobutyric acid HCl) is a neuropsychotropic drug that was discovered and introduced into clinical practice in Russia in the 1960s. It has anxiolytic and nootropic (cognition enhancing) effects. It acts as a GABA-mimetic, primarily at GABA(B) and, to some extent, at GABA(A) receptors. It also stimulates dopamine receptors and antagonizes beta-phenethylamine (PEA), a putative endogenous anxiogenic. The psychopharmacological activity of phenibut is similar to that of baclofen, a p-Cl-derivative of phenibut. This article reviews the structure-activity relationship of phenibut and its derivatives. Emphasis is placed on the importance of the position of the phenyl ring, the role of the carboxyl group, and the activity of optical isomers. Comparison of phenibut with piracetam and diazepam reveals similarities and differences in their pharmacological and clinical effects. Phenibut is widely used in Russia to relieve tension, anxiety, and fear, to improve sleep in psychosomatic or neurotic patients; as well as a pre- or post-operative medication. It is also used in the therapy of disorders characterized by asthenia and depression, as well as in post-traumatic stress, stuttering and vestibular disorders.

Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug - PubMed
Phenibut (beta-phenyl-gamma-aminobutyric acid HCl) is a neuropsychotropic drug that was discovered and introduced into clinical practice in Russia in the 1960s. It has anxiolytic and nootropic (cognition enhancing) effects. It acts as a GABA-mimetic, primarily at GABA(B) and, to some extent, at...
Phenibut (β‐Phenyl‐GABA): A Tranquilizer and Nootropic Drug - PMC
Phenibut (β‐phenyl‐γ‐aminobutyric acid HCl) is a neuropsychotropic drug that was discovered and introduced into clinical practice in Russia in the 1960s. It has anxiolytic and nootropic (cognition enhancing) effects. It acts as a GABA‐mimetic, ...
- Status
- Not open for further replies.