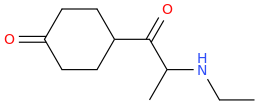
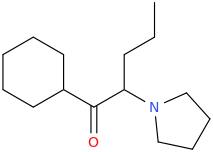
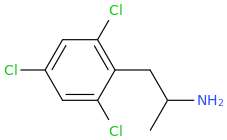
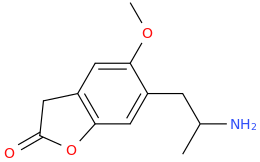
You still have not backed up your claim to be in drug design. It still has absolutely zero substance WHATSOEVER; and given your repetitive posting of utter, total garbage about drugs that all you have been able to do in any such instance, is to 'assign' a trivial name to the structure devoid of meaning, prancing about as if you are Sasha Shulgin himself, whilst being able to give not ONE trip report. If you were anything but a liar,regarding being a 'drug designer' then you would have actually MADE, and tested these garbage molecules, and given us trip reports as feedback. Being a drug designer. well, it might surprise you Dresden; but it involves actually designing drugs. Drawing pictures, deciding what name you will pull from out of your arse and not even making educated reasoning as to WHY these specific molecules have been drawn; what activity you expect they would have and why doesn't make you a 'drug designer'.
Tell us, just how many of these compounds you have actually synthesized, how many have undergone biossay? or am I right in my belief that you have, in reality, not synthesized a single one of them much less ever tasted, and that all you have ever done is depict structures using chemdraw or similar and given them meaningless names.
The way you have acted, posting this meaningless dreck, that justifies our asking you to back up your claims. So, back up your lofty claims, or admit to us all, that you are a liar and stop with the posting of this damnable, disgraceful spammy and meaningless garbage. I find it absolutely unbelievable that this spamming of total fucking shit and lies has been allowed in ADD. Its fucking shocking to be honest, that this GARBAGE has been allowed to continue.
So, the time for you to prove the truth of your words, or for you to admit to us all that you are, in actual fact, telling us all lies and stop the shitspeak.