Bagseed
Bluelighter
the discussion is about the diarylmethoxy carbon, not the tropan
N&PD Moderators: Skorpio | someguyontheinternet
You are right, but my point was: chirality is drawn if it is relevant, and at that stage of consideration it is only very generally shown that there are possible substitutions that are not necessarily equal but they may be equal or not present at all. There is nothing said of what they may be, so it seems irrelevant or premature to point out that there are potential chirality issues there. I think, like hydrogens it is not drawn until it really becomes a relevant matter. Anyway that's my take on it. Even if the chirality is not ambiguous anymore it is not necessarily drawn, unless it matters in the context. Look at the alpha carbon of amphetamines. Often enough ignored, cause it is not always significant in all matters.
Sorry for introducing confusion. You are right that diphenylmethoxy- part is NOT chiral, due to having two identical (phenyl) group. It is just prochiral, The questions about chirality I asked is for in-case that the phenyls are asymmetrically substituted. (Eg, one Phenyl plus one chlorophenyl) from that jellyfish-like molecule
yes, then it'd be chiral because the quaternary carbon that bridges the two would have 4 differing substituents.






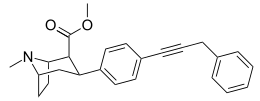
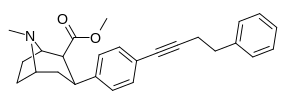




wtf is this endogenous ligand's structure: https://www.ncbi.nlm.nih.gov/pubmed/2887250 for the PCP site?
Are all endogenous ligands in the brain basically 'designed' to not pass the BBB? I mean: are there endogenous brain neurotransmitters known that could be active when administered, is the BBB made to transport some compounds unilaterally or would you never find any such administerable drug because it would leak out of the brain if it could also get in there / or get metabolized really quickly before it could get anywhere?
