aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
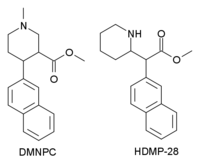
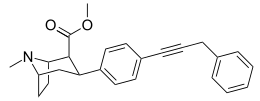
^ Synthetically the difference between phenyltropanes and those 4-aryl-3-carboalkoxypiperidines is huge IMO. I don't know if there are any short-acting phenyltropanes, but if we limit ourselves to the simplest 2-carboalkoxy-3-aryltropanes, they are all long-acting stimulants which most possibly makes them poor recreational drugs which combined with not necessarily cheap common substrate makes them not attractive as "RC's".
The piperidines might as well be more like SSRI/SNRI antidepressant with little recreational value, they might have been tested in small groups of volunteers and turned out not interesting.
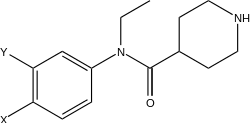
They are only long lasting because of loss of ester functionality. Bridge the piperidine with the aryl ring with an ester to reduce half life.
Compared to cocaine itself, the syntheses of these piperidines would be much, much easier. They look quite reasonable to synthesise and the data for some of them makes it look much more potent than cocaine itself.