Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
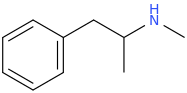
I have a hunch this one would be better due to greater ring activation afforded by the added methoxy group:

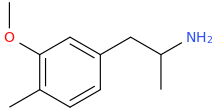
However, I was completely wrong about 3-MeO-MA, which is almost devoid of psychoactivity (I actually tried it.).

However, this one is known to be active:
* * *
We can dance if we want it. We can go to a place they'll never find. Because your friends don't dance, and if they don't dance, well they're no friends of mine. As long as we have music, everything will work out fine.
* * *
This

is probably a safer bet, as N-methylation makes these compounds weaker and smoother.
And I seriously doubt that

is a reuptake inhibitor.
This

might be one though.
* * *
Bad seed from bad sperm.
Herb got my wig fried like a bad perm.

However, I was completely wrong about 3-MeO-MA, which is almost devoid of psychoactivity (I actually tried it.).

However, this one is known to be active:

* * *
We can dance if we want it. We can go to a place they'll never find. Because your friends don't dance, and if they don't dance, well they're no friends of mine. As long as we have music, everything will work out fine.
* * *
This

is probably a safer bet, as N-methylation makes these compounds weaker and smoother.
And I seriously doubt that

is a reuptake inhibitor.
This

might be one though.
* * *
Bad seed from bad sperm.
Herb got my wig fried like a bad perm.
Last edited: