-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide -
N&PD Moderators: Skorpio | someguyontheinternet
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Midnight Sun
Bluelighter
what would happen to that bottom one metabolically? would that ring open right away?
Isobenzofuran is so unstable I don't think it could even be made
Dresden, if n-ethyls aren't prodrugs then any N-ethyl DOx/2C-X would be a no go, based simply off what we know about simple n-methylation
Seeing as there are plenty of cases of bulky n-substituents being readily cleaved (n-benzyl, n-lysine, etc etc) I would still lean towards n-ethyl following suit
belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
No, it wouldn't. I don't anything would happen to it metabolically. For example, the related tetrahydrofuran (THF) is a known and stable compound:

THF (2,3,4,5-tetrahydrofuran)
THF is stable, as far as ethers go anyway, but not metabolically. It is metabolized into GBL (in two steps), albeit quite slowly, but it is metabolized nonetheless.
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
Ok, so LOOPY is dead on arrival. I get it.
IIRC N-propylamphetamine ('prop') [Schedule I]

is metabolized into this

Don't know about eth's metabolites:

As for N-ethyl-DOx's, I would be surprised if their metabolites have been studied yet by anyone at all.
As for me, I think DOAM would be fun, largely based on its effects' description in PiHKAL:

Alexander Shulgin objected to its low relative potency, but 10mg (or even 20 or 30) doesn't sound like too much material for a dose to me.
Is DOAM a possible CB agonist? The description in PiHKAL sounds like being stoned to me.
Speaking of amyl's, wouldn't it be great if these two beauties are active and unscheduled?

TAMMY
and

AMY
They look like some sort of surfactant adjuvants.
And I have always wanted to try
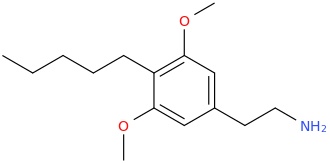
as well. I know that

was once a (prescription, I believe) drug in France, for all that it's worth.
IIRC N-propylamphetamine ('prop') [Schedule I]

is metabolized into this

Don't know about eth's metabolites:

As for N-ethyl-DOx's, I would be surprised if their metabolites have been studied yet by anyone at all.
As for me, I think DOAM would be fun, largely based on its effects' description in PiHKAL:

Alexander Shulgin objected to its low relative potency, but 10mg (or even 20 or 30) doesn't sound like too much material for a dose to me.
Is DOAM a possible CB agonist? The description in PiHKAL sounds like being stoned to me.
Speaking of amyl's, wouldn't it be great if these two beauties are active and unscheduled?

TAMMY
and

AMY
They look like some sort of surfactant adjuvants.
And I have always wanted to try

as well. I know that

was once a (prescription, I believe) drug in France, for all that it's worth.
Last edited:
pharmakos
Bluelighter
re: DOx's with long 4-alkyl chains... the 5HT binding affinity actually goes up as the chain gets longer, even butyl and beyond... but they aren't active psychedelics, so its likely that they're either antagonists or partial agonists.
SKL
Bluelight Crew
- Joined
- Sep 15, 2007
- Messages
- 14,632
Dresden, propylamphetamine is a very nice relatively mild stimulant, used to circulate as an RC, I used a lot of it in smallish doses. It would be a good pharmaceutical for AD(H)D. Amfepentorex is definitely an interesting molecule. Was used for a bit for appetite suppression, like a hundred other weird stimulants I take it.
DOx based 5HT antagonists might actually be useful therapeutic drugs, e.g., in treatment of serotonin syndrome, as the sometimes used cyproheptadine* is a much dirtier drug (anticholinrgic, etc.)
They may even have psychiatric uses. A lot of the new atypicals have 5HT antagonism and isolating it might be a worthwhile exercise.
* cyproheptadine, something of an interesting molecule in it's own right:

re: DOx's with long 4-alkyl chains... the 5HT binding affinity actually goes up as the chain gets longer, even butyl and beyond... but they aren't active psychedelics, so its likely that they're either antagonists or partial agonists.
DOx based 5HT antagonists might actually be useful therapeutic drugs, e.g., in treatment of serotonin syndrome, as the sometimes used cyproheptadine* is a much dirtier drug (anticholinrgic, etc.)
They may even have psychiatric uses. A lot of the new atypicals have 5HT antagonism and isolating it might be a worthwhile exercise.
* cyproheptadine, something of an interesting molecule in it's own right:

Nagelfar
Bluelight Crew

Another attempt at a opioidergic-MAT reuptake inhibitor a la methylphenidate/fentanyl, using a reverse ester of the beta-carbamoyl cocaine analogue to fit the nitrogen into the proper place in the bridge for the fentanyl overlay. Keeping the MPH orientation of the carbmethoxy probably just makes it an antichlorinergic, however, like tematropium
pharmakos
Bluelighter
* cyproheptadine, something of an interesting molecule in it's own right:

interesting, never saw that stuff before. reminds me that cyclobenzaprine is a 5HT-2A antagonist -- a fact that i learned long ago and forgot about.
it is interesting how some tricyclics have stood the test of time, while most of the rest of that class is long forgotten.
SKL
Bluelight Crew
- Joined
- Sep 15, 2007
- Messages
- 14,632
So is there anything interesting we can do with rimonabant, a CB1 antagonist. I have reliable reports of it's being useful in treating overdoses of synthetic cannabinoids. It was used as an appetite suppressant, like half of the other things in this thread (lol), but it's been discontinued, stateside, though, as it had some unfortunate side effects. Those of us working in clinical psych during the NYC "spice/K2 epidemic" would've liked to have a parenteral formulation of this stuff on hand, believe me.
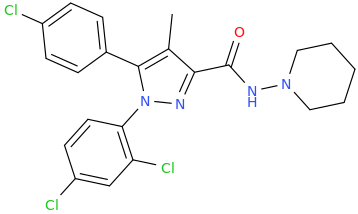
Novel 3-substituted rimonabant analogues lack Δ(9) -tetrahydrocannabinol-like abuse-related behavioural effects in mice, but have some "cannabimetric" effects, claimed that they are suggested to lack abuse potential, whatever that means . The first two "... neither substituted for nor antagonized THC's discriminative stimulus [in mice.]"

Exploring the binding features of rimonabant analogues and acyclic CB1 antagonists: docking studies and QSAR analysis, other interesting stuff, with a bunch of other CB antagonists.

Novel 3-substituted rimonabant analogues lack Δ(9) -tetrahydrocannabinol-like abuse-related behavioural effects in mice, but have some "cannabimetric" effects, claimed that they are suggested to lack abuse potential, whatever that means . The first two "... neither substituted for nor antagonized THC's discriminative stimulus [in mice.]"

Exploring the binding features of rimonabant analogues and acyclic CB1 antagonists: docking studies and QSAR analysis, other interesting stuff, with a bunch of other CB antagonists.
Last edited:
belligerent drunk
Bluelight Crew
- Joined
- Sep 16, 2015
- Messages
- 3,482
Ok, so LOOPY is dead on arrival. I get it.
Not necessarily. THF is quite different from "LOOPY" just by size. LOOPY may not fit into the enzyme which oxidizes THF. I mean, just because simple alcohols get destroyed by ADH doesn't mean any compound containing a hydroxyl group would have the same fate. But then again there's always the possibility.
Anyway, I actually just commented on THF. It's mostly stable on a shelf if stabilized, but not so in the body. I actually tried to get off on THF a few times just out of pure curiosity because an article described a metabolic pathway leading to GBL/GHB. As far as I'm concerned it worked (5ml of THF), but it was disgusting to drink even in like 5% dilution and my stomach didn't like it. Now I stick to dissolving things with it, it's an excellent solvent.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Isobenzofuran is so unstable I don't think it could even be made
Dresden, if n-ethyls aren't prodrugs then any N-ethyl DOx/2C-X would be a no go, based simply off what we know about simple n-methylation
Seeing as there are plenty of cases of bulky n-substituents being readily cleaved (n-benzyl, n-lysine, etc etc) I would still lean towards n-ethyl following suit
What's an example of n-benzyl being cleaved? You referring to benzphetamine? Couldn't find any pharmacokinetic for said compound. I think cleavage of n-lysine is a different process (if you are referring to cleavage of lysine from vynase to give dexamph) as a peptide bond is being broken rather than just a N-C bond. Mechanism will be somewhat similar to how chymotrypsin breaks down peptides I think.
https://en.wikipedia.org/wiki/Catalytic_triad
However I do think n-ethyl will be cleaved.
Last edited:
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212

bk-DMT

'Stoned' bk-DMT
And of course, all the requisite 4-OH's and 5-MeO's.
I really think that bk-DMT is a good idea. Its carbonyl group is borrowed from the now infamous JWH's. The pentyl and the carbonyl in 'Stoned' bk-DMT are too; however, out of these 6 new molecules, bk-DMT piques my interest the most.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
I know that

was once a (prescription, I believe) drug in France, for all that it's worth.
What's this one called?
SKL
Bluelight Crew
- Joined
- Sep 15, 2007
- Messages
- 14,632
Ancient alt.drugs post archived on Erowid, on caffeine analogs
Probably not really viable recreational drugs (where does the methylenedioxy go? ), but interesting and underlooked chemistry.
), but interesting and underlooked chemistry.
Some of the more interesting ones:
Allegedly these two are CNS depressants:


Super-caffiene, ~100x potency at A1/A2:

This guy is used as a pesticide:

There are loads more, and a good bit of research since then on the subject which I'm just sort of delving into now for shits and giggles. I'm snagging a few papers. Could post more if I find interesting stuff and people are interested.
Probably not really viable recreational drugs (where does the methylenedioxy go?
Some of the more interesting ones:
Allegedly these two are CNS depressants:


Super-caffiene, ~100x potency at A1/A2:

This guy is used as a pesticide:

There are loads more, and a good bit of research since then on the subject which I'm just sort of delving into now for shits and giggles. I'm snagging a few papers. Could post more if I find interesting stuff and people are interested.
Last edited:
Midnight Sun
Bluelighter
No, I said LOOPY was DOA b/c Midnight Sun stated that isobenzofuran is super unstable, not because of anything having to do with the THF discussion.
It likes to polymerize... per wiki apparently if you chill the fuck out of it, it'll stabilize enough to work with, so maybe someone out there dedicated enough could figure something out, but the Chinese are so lazy they don't even like synthesizing 6-xapb so I wouldn't get my hopes up. The MDxx derivative clock is kind of starting to run out, I'm curious as to what they'll push out next
Maybe they'll try to unload the rest of their postban APB stock as N-OH-5-MAPB, that wouldn't surprise me a bit
What's an example of n-benzyl being cleaved? You referring to benzphetamine? Couldn't find any pharmacokinetic for said compound. I think cleavage of n-lysine is a different process (if you are referring to cleavage of lysine from vynase to give dexamph) as a peptide bond is being broken rather than just a N-C bond. Mechanism will be somewhat similar to how chymotrypsin breaks down peptides I think.
https://en.wikipedia.org/wiki/Catalytic_triad
However I do think n-ethyl will be cleaved.
Yeah I was referring to benzphetamine or that atrocity benzedrone, but matter of fact I'll even lump NBOMes in with them. you are right about lysine coming off in a different manner, isn't that done in the bloodstream?
pharmakos
Bluelighter
i'm definitely interested in caffeine analogues, Mr. K. Love. caffeine is arguably the greatest drug their is when looked at in terms of cost vs. benefit. improving on it would be wonderful.
- Status
- Not open for further replies.




