Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
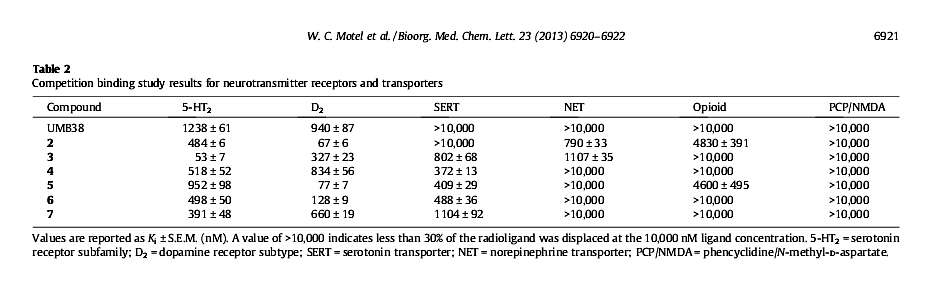
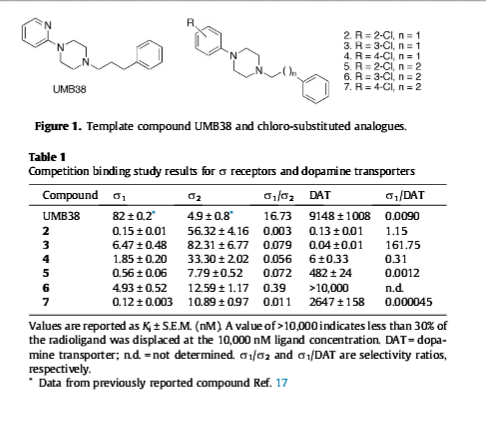
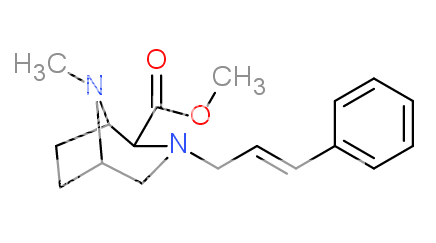
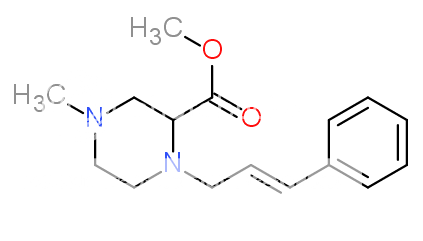
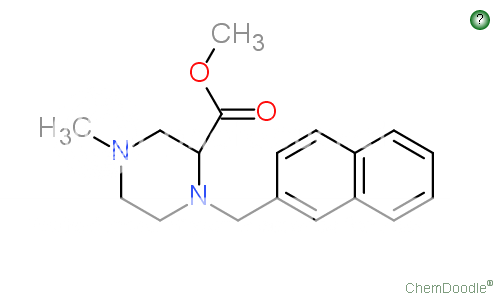
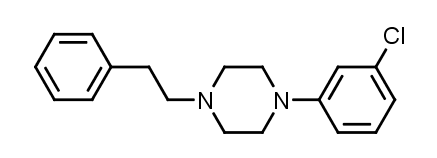
1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine
DAT dissociation constant (Ki = 0.04 nM)
NET (Ki = 1107 nM )
SERT (Ki = 802 nm)
"10,000 times more potent than cocaine as a dopamine transporter inhibitor in vitro"
...and look how close it is to some of the fastest onset, short duration, opioid analgesics(!)
Fentanyl:
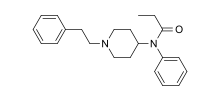
MT-45:
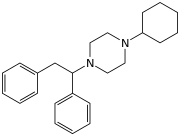

DAT dissociation constant (Ki = 0.04 nM)
NET (Ki = 1107 nM )
SERT (Ki = 802 nm)
"10,000 times more potent than cocaine as a dopamine transporter inhibitor in vitro"
...and look how close it is to some of the fastest onset, short duration, opioid analgesics(!)
Fentanyl:

MT-45: