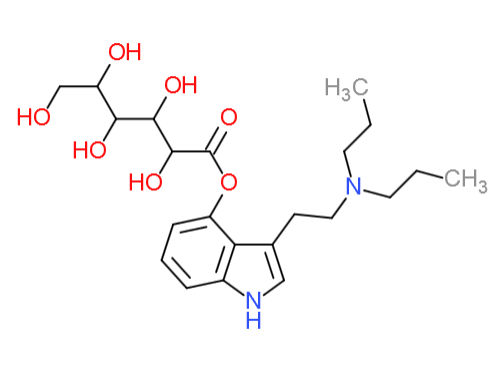
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Pyridinyl amps not in PiHKAL.
Not only can the DMT overlay skeleton be found in LSD, but the methamphetamine (2-methylamino-1-phenylpropane) one as well.
Good points. Thanks for checking PIKHAL.
I met Alec Shulgin at an ACS convention in Anaheim, CA years ago. Nice guy. Wish I had had more time to spend with him.
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Can anyone give any info as to both acute and longterm toxicity of pthalimide? (unrelated to the above, it just reminded me)
Because of the instability of primary amine cathinones, its possible to employ a pro-drug strategy, namely preparing the pthalimidopropiophenone homolog to the cathinone, the pthalimide getting cleaved off in the acidic environment of the stomach, thus causing the otherwise unstable as hell psychedelic cathinone analogs to be released into an environment which ensures they are dilute in solution and ergo, less likely to meet up with another molecule of the psychedelic cathinone and dimerize to pyrazine crap.
I'd very much like to know how safe this is, not interested for simple stimulant and thus heavy use, but for occasional use in facilitating research of cathinone analogs of psychedelic phen/phets.
Because of the instability of primary amine cathinones, its possible to employ a pro-drug strategy, namely preparing the pthalimidopropiophenone homolog to the cathinone, the pthalimide getting cleaved off in the acidic environment of the stomach, thus causing the otherwise unstable as hell psychedelic cathinone analogs to be released into an environment which ensures they are dilute in solution and ergo, less likely to meet up with another molecule of the psychedelic cathinone and dimerize to pyrazine crap.
I'd very much like to know how safe this is, not interested for simple stimulant and thus heavy use, but for occasional use in facilitating research of cathinone analogs of psychedelic phen/phets.
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Yeah, that was where I got the idea from, I recall something about pthalimidopropiophenone being used orally as a stimulant in, IIRC, israel.
I think it was israel, anyway, I definitely recall something about it being done.
Just want to know about the potential toxicity of pthalimide, since if is highly toxic obviously my research will have to go in other directions.
But it'd be very good to explore more beta-ketophenthylamines and psychedelic cathinones without that damnable instability. In the case of cathinones, the alpha-methyl group provides some additional stability doesn't it? how much greater is the stability of a given beta-ketophenethylamine vs it's alpha-methyl homologue?
Psychedelic cathinones have interested me for a while, as has potentially lengthening the chain, see if the corresponding pyrovalerones etc. are effective as psychedelics.
I think it was israel, anyway, I definitely recall something about it being done.
Just want to know about the potential toxicity of pthalimide, since if is highly toxic obviously my research will have to go in other directions.
But it'd be very good to explore more beta-ketophenthylamines and psychedelic cathinones without that damnable instability. In the case of cathinones, the alpha-methyl group provides some additional stability doesn't it? how much greater is the stability of a given beta-ketophenethylamine vs it's alpha-methyl homologue?
Psychedelic cathinones have interested me for a while, as has potentially lengthening the chain, see if the corresponding pyrovalerones etc. are effective as psychedelics.
Neopunk
Bluelighter
- Joined
- Jun 16, 2018
- Messages
- 1,254
Why do you want to know about the toxicity of phthalimide, when by cleavage of the prodrug not phthalimide, but phthalic acid is obtained? The first one however is quite non-toxic. Once I performed a Gabriel-synthesis and remember it not even having H&P-statements :D
Of course that doesn't mean it's safe in long-term use. But really, you rather have to look at phthalic acid, which seems to be irritating to skin, eyes and airways.
My guess would be that the use of a phthalimido-protecting group shoulnd't be too problematic if used occasionally.
Of course that doesn't mean it's safe in long-term use. But really, you rather have to look at phthalic acid, which seems to be irritating to skin, eyes and airways.
My guess would be that the use of a phthalimido-protecting group shoulnd't be too problematic if used occasionally.
Last edited:
well I guess you need the electronegative substitution (oxygen) on the benzene ring, dunno if a carbon on there will work.
3-Methoxy-4-methylamphetamine is definitely entactogenic.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Would it be feasible to make an ester prodrug that has OH groups to increase water solubility?
Nature beat you to it... it's the phosphoric acid ester known as psilocybin.
BPD help plz
Bluelighter
- Joined
- Sep 27, 2018
- Messages
- 229
I Bow Before You Oh Masterful one!!Wee! Great!
So now the random molecule wont spread around all over the topics!
Let me start this (supposedly fun?) topic by this one:
(Please vote a name for it!)

BPD help plz
Bluelighter
- Joined
- Sep 27, 2018
- Messages
- 229
Hi, errr...I'm a newbee here...first day and all....i have lots of 'practical' knowledge over the years, sadly the chemistry side of things i failed to ever pay attention to therefore alas my first time try of making methcathinone went totally wrong.....i'm kinda at a loss of how to 'blog' post...but mucho impressed at your structure knowledge, is it ok if i hang on your coat tails a bit?
Be no worries, you know every people is clueless before then learn something!
Just take it step by step and not just hop to the fancy stuffs without understanding the basics of what is going on.
One big difference between “cooker” and “chemist” is that cooker only follow the recipes blindlessly with no understanding what the molecules is behaving inside the reaction, while the chemist have knowledge and can “see” what, how, when, why and where things happens.
This topic is meant to be fun, most things are designed out of some basis but they arent tested out so never make them and try. That will be extremely dangerous. Some of them are just for mental masturbation like my MDBuckyball you quoted!
Just take it step by step and not just hop to the fancy stuffs without understanding the basics of what is going on.
One big difference between “cooker” and “chemist” is that cooker only follow the recipes blindlessly with no understanding what the molecules is behaving inside the reaction, while the chemist have knowledge and can “see” what, how, when, why and where things happens.
This topic is meant to be fun, most things are designed out of some basis but they arent tested out so never make them and try. That will be extremely dangerous. Some of them are just for mental masturbation like my MDBuckyball you quoted!
BPD help plz
Bluelighter
- Joined
- Sep 27, 2018
- Messages
- 229
I wish my Science teacher had been remotely interesting then i may have actually gone done the chemistry path as i recall 'asking santa' for a chemistry set......a MASSIVE chemistry set (for kids but i bet you couldn't get them now a-days)...and santa....(with my Dad's help)...got me said chemistry set.....it , as i recall, was equipped with the works, test tubes, chemicals of all sorts ...over 1000 different 'experiments'.....think i did about 1 with Dad on Xmas day before it went into storage before moving house then never saw it again.....but by then....i had started Science and my teacher was as interesting as watching paint dry....actually, no, i think i learnt more watching paint dry
As of current state, there are enough online resource that can be used for self learning comparing to old days.
Trust me, learning in most school, univ is just as boring as what you described.
Chem has much more, just not skip to learn only the “cool” things only.
You dont even need an interactive media to be fluent in chemistry, just a scan of a good textbook will do
Some are not too hard to understand thoroughly. You just need to know where to download them (for free)
It is ok if textbooks are of old age, you just need to update your knowledge when you find a more recent source.
I can speak of this by first hand experience, now being a chemist myself, i learn univ-grade chem since like 14-15yo
By just sifting thru the “offline” library in school, univ. And, more importantly, being as chemistry obsession since a child,
I still do find how chem is taught in school super boring, and in univ just....er...okay-level.
Query me in pm if you still want sth specific to learn Let’s not flood this thread with personal re-re-reply
Let’s not flood this thread with personal re-re-reply
Trust me, learning in most school, univ is just as boring as what you described.
Chem has much more, just not skip to learn only the “cool” things only.
You dont even need an interactive media to be fluent in chemistry, just a scan of a good textbook will do
Some are not too hard to understand thoroughly. You just need to know where to download them (for free)
It is ok if textbooks are of old age, you just need to update your knowledge when you find a more recent source.
I can speak of this by first hand experience, now being a chemist myself, i learn univ-grade chem since like 14-15yo
By just sifting thru the “offline” library in school, univ. And, more importantly, being as chemistry obsession since a child,
I still do find how chem is taught in school super boring, and in univ just....er...okay-level.
Query me in pm if you still want sth specific to learn
The license period on my copy of ChemOffice 17 expired. Even though I own the earlier 11 version, that's for 32-bit machines & won't work in my Dell 64-bit Windows 7 desktop.
Perkin-Elmer wants over $2900 for ChemOffice 17. Even with the 20% discount they will give me for owning the earlier version, that's still $2500 or so for a program I only use occasionally.
I searched eBay for ChemOffice & found a guy in Spain selling ChemOffice 16 which is Windows 7 compatible. New, with download & license key. Listing #232946706683. For under $5. Three left. My experience with both versions 11 & 17 showed that they were basically the same structure drawing version of ChemDraw. I bought one. He has 3 left. Great value for $5!
Perkin-Elmer wants over $2900 for ChemOffice 17. Even with the 20% discount they will give me for owning the earlier version, that's still $2500 or so for a program I only use occasionally.
I searched eBay for ChemOffice & found a guy in Spain selling ChemOffice 16 which is Windows 7 compatible. New, with download & license key. Listing #232946706683. For under $5. Three left. My experience with both versions 11 & 17 showed that they were basically the same structure drawing version of ChemDraw. I bought one. He has 3 left. Great value for $5!
Last edited:
- Status
- Not open for further replies.