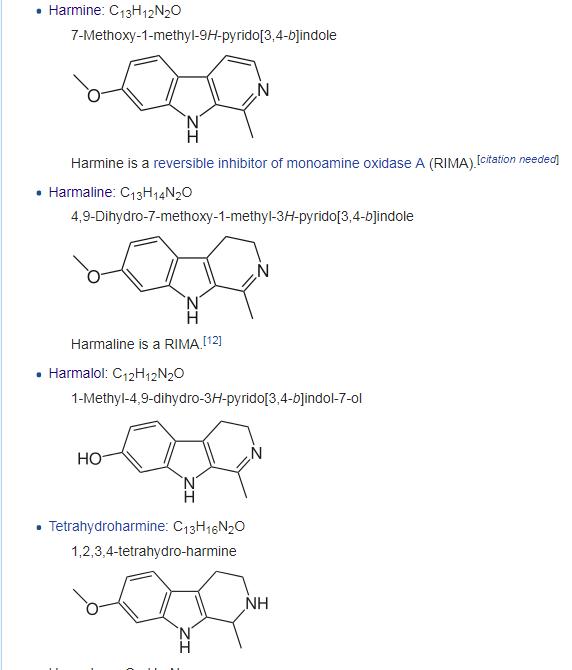
Unlike the Carbolines such as Harmine and the dihydrocarbolines (like Harmaline and Harmalol) that are MAO inhibitors, the tetrahydrocarbolines (THBCs) like tetrahydroharmine or pinoline are not. Pinoline is about 1000x less as MAO inhibitor than the monounsaturated and fully oxidized carbolines (will dig out refs on that later).
On the other hand, iirc unlike the THBCs, carbolines and dihydrocarbolines are not monoamines releaser/reuptake inhibitor ??? correct me if I am wrong tho. I cant find any study on that. Almost all focus on their MAO activity They look similar (chemical structure wise) but they have distinct pharmacology. I would think THBCs would be more like DMT but apparently they're also somehow like MDMA I mean in terms of serotonergic/da/net releasing ratios.. So possibly they'll be like a DMT/MDMA combination in effect.. but who knows? .. oh BTW, as I mentioned they're robustly neurogenic like LSD, DOM or noribogaine .. I mean pinoline but not harmaline or harmine.
edit: that woman who OD'ed on Syrian rue harmala was actually lucky. She probably was not taking any other medication (and/or foods like cheese) containing groups (amines) that are detoxify by MAO. If she's eaten cheese (containing Tyramine) MAO inhibition might shoot up concentration of its brain tyramine and all sort of amines. Cheese might get her pretty high, I mean psychotic high because of the tyramine. But could be brain dead or worse..