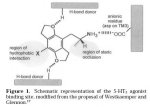
Simple question, why is 2C-TFM so potent?
For what I have understant, the molecule is more powerfull if the 4-substituent is more lipophilic (and if it's not too big, for steric reason). Right?
So, i quote the wiki:
"The trifluomethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine"
So, the polarity of trifluoromethyl is bigger than chlorine! So, 2C-TFM is less lipophilic than 2c-c! It must be less potent... But it's not the case! WHY?!
Is something special with 2C-TFM to cross the BBB?
Or the molecule bind to the receptor in a different way?
Maybe, the characteristics of a trifuloromethyl is different in a benzene ring?
Thank you for your answer!
Sorry for my english, hope you understant me!
And, sorry if the question is stupid... I make a search but don't find an answer...
Good day!
For what I have understant, the molecule is more powerfull if the 4-substituent is more lipophilic (and if it's not too big, for steric reason). Right?
So, i quote the wiki:
"The trifluomethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine"
So, the polarity of trifluoromethyl is bigger than chlorine! So, 2C-TFM is less lipophilic than 2c-c! It must be less potent... But it's not the case! WHY?!
Is something special with 2C-TFM to cross the BBB?
Or the molecule bind to the receptor in a different way?
Maybe, the characteristics of a trifuloromethyl is different in a benzene ring?
Thank you for your answer!
Sorry for my english, hope you understant me!
And, sorry if the question is stupid... I make a search but don't find an answer...
Good day!