MedicinalUser247
Bluelighter
- Joined
- Aug 2, 2023
- Messages
- 803
This just makes me wonder how euphoric Diacetylmorphone would be.
N&PD Moderators: Skorpio | thegreenhand
There are 3 main opioid receptors... mu, kappa, delta.
These receptors also have sub-receptors, such as mu1, mu2, mu3.
Most opioids bind to mu, but they don't all bind to the sub receptors.
I believe it's mu2 that is responsible for the warm euphoria people are generally after.
Although agonizing mu in general is going to give some effect.
Fentanyl for example, is 50x "stronger" than morphine & heroin, but it doesn't actually feel better because it probably doesn't bind to mu2 or have a binding profile that is favorable for euphoria at all.
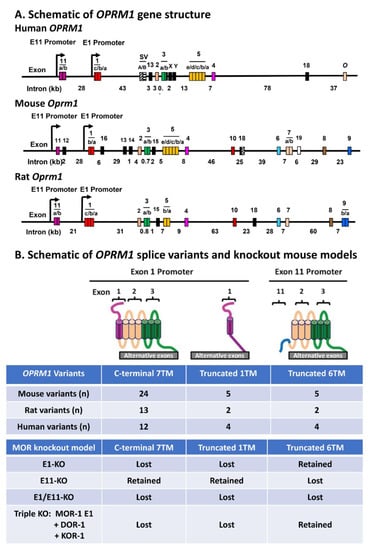
is there any data backing out the pharmocological characteristics of ligands specifically agonizing the 10 or more known splice variants of MOR?
There is only one gene for mu the receptor variation is by posttranslational splicing, so these are not really sub receptors but modifications of the prototype receptor either way they add a layer of complexity that is not widely appreciated or understood.
In particular euphoria va mu2 splice variant agonism?
For certain hitting one splice variant over another one has the potential to separate some of the the different effects of general mu agonism, though from a pharma development view euphoria is an undesirable off target effect, in general pharma would prefer dull grey painkiller opioids over effective but euphoric compounds.
The existance of these splice variants has been known since the early 2000s the need of some opioids to interact with specific splice vairants to have any effect is known, but I am not aware of work that has neatly correlated interaction with one splice variant and specific effects, like euphoria.
targetting splice variants:

Exploring Pharmacological Functions of Alternatively Spliced Variants of the Mu Opioid Receptor Gene, Oprm1, via Gene-Targeted Animal Models
The mu opioid receptor has a distinct place in the opioid receptor family, since it mediates the actions of most opioids used clinically (e.g., morphine and fentanyl), as well as drugs of abuse (e.g., heroin). The single-copy mu opioid receptor gene, OPRM1, goes through extensive alternative...www.mdpi.com
Yeah, I'm a highschool dropout, but have been doing drugs for 27+ years. And pharmacology became a huge interest of mine & I think it actually helped keep me safer.IMHO the NIH paper is sadly complete garbage, the various Mu subtypes aren't even objectively defined. This is written by a pair of MDs making noise (remember how little pharmacology MDs study) Practicing MDs are pretty much the least knowledgeable about pharmacology. Their level of knowledge gets beaten every time by junkies, often by barristas too. The second paper is pretty poor, and these two cannot be the sum of human knowledge on the subject, so there must be more.
If I get time I will have a look and see if there is any recent literature written by pharmacologists not MDs, MDs should stick to what they know and are good at: scamming insurance companies, making soothing sheep like noises and avoiding taking blame or responsibility.
There is something to the complexity with mu, it seems though that the various subtypes haven't been categorized or studied properly. I would like to see the splice variants defined by AA sequence and then the various ligand affinity for each splice then from that the pharmacology can be guessed at.
A lot of those differences could be due to biased agonism. All of the opioid receptors can either couple to a G protein for the canonical pathway, or couple to beta arrestin and signal to different targets.Yeah, I'm a highschool dropout, but have been doing drugs for 27+ years. And pharmacology became a huge interest of mine & I think it actually helped keep me safer.
Although I'm still just an amateur & I definitely can only superficially touch on most subjects (hey I'm trying though right).
If you find any more info, I'd love to read it though! Cause I'm very curious.
My opioid use over the past 17 years has involved tramadol, codeine, hydrocodone, oxycodone, methadone, buprenorphine, diacetylmorphine, propoxyphene, loperamide, kratom (which I don't really consider an opioid anyway), fentanyl, etc..
And I'd say from my subjective experience, every single one of those opioids all felt subjectively different from one another. Even though they're all mu agonists.
Heroin was definitely way more euphoric than fentanyl, even though fentanyl is 50x stronger. So it seems like the mu receptor sub type stuff could be plausible. Fentanyl was much more sedating than heroin to me too (although much shorter lasting) yet it had almost no euphoria or feeling of well being. Seems odd if euphoria is strictly based on any mu receptor. There has to be more at play that makes one opioid more euphoric or enjoyable than another, even when they're both full mu agonists.
Interesting.A lot of those differences could be due to biased agonism. All of the opioid receptors can either couple to a G protein for the canonical pathway, or couple to beta arrestin and signal to different targets.
This concept is pretty hot rn (despite some pretty big hiccups in the early days of it), as it provides a pretty good explanation of why some opioids are more euphoric and why other opioids tend to suppress breathing more.
Originally, beta arrestin was only known as a molecule that mediates internalization of active G-protein coupled receptors. When a receptor is activated (the amount depends on the receptor), it will be phosphorylated to produce a binding site for beta arrestin, which recruits endocytosis machinery to suck the receptor into a cell, where it was thought to be inactive. Controlling the amount of receptors on the cell surface was thought of as a way the body mediates tolerance.Interesting.
I remember reading a bit about beta arrestin. It has something to do with tolerance too doesn't it?
I think it was buprenorphine that I was reading about where it was mentioned.
Now I'm curious.
What exactly is a canonical pathway? If you don't mind explaining...
A lot of human hormone release is entrained to circadian rhythms, so the time of dosage can definately change the effects of a drug.Speaking of buprenorphine...
I've noticed in the past 7 years that taking buprenorphine at night time makes me feel more "high" & stimulated. A tad more pain relief too.
But taking buprenorphine when I wake up & during the day lacks much feeling at all, other than being tired.
Is it possible for the subjective effects of drugs to feel different depending on the time of day? And if so, why? Is it because the balance of neurochemicals in our brains is different depending on if it's day or night time? Is this even plausible?
Thanks for clarifying!Originally, beta arrestin was only known as a molecule that mediates internalization of active G-protein coupled receptors. When a receptor is activated (the amount depends on the receptor), it will be phosphorylated to produce a binding site for beta arrestin, which recruits endocytosis machinery to suck the receptor into a cell, where it was thought to be inactive. Controlling the amount of receptors on the cell surface was thought of as a way the body mediates tolerance.
Recently, it has been discovered that beta arrestin doesn't just scaffold endocytosis machinery, but also scaffolds signaling enzymes. These enzymes follow the internalized receptor and signal as it is being dragged to the endoplasmic reticulum.
To oversimplify, G-protein signaling either increases or decreases cyclic AMP production (Gs or Gi respectively), or it can cause calcium signaling (Gq). Some receptors like opioid receptors can also open ion channels (GIRK channels).
Beta arrestin signaling tends to converge on the MAP kinase pathway, engaging a totally different set of enzymes to achieve different results.
I just refer to the general G-protein pathway as canonical, as that is what was first discovered and is broadly taught about.
A lot of human hormone release is entrained to circadian rhythms, so the time of dosage can definately change the effects of a drug.
I remember reading that one aspect of opioid tolerance is that the body releases a good amount of norepinephrine when it expects to be high (for example this would occur when somebody is fixing up for IV use). This effect is dependant on context, and occurs when using in a location where you usually use. People using in new environments will have less of this effect, and are therefore more susceptible to overdose.
Fentanyl is considered to be a an opioid that has a high ratio of beta arrestin signaling to g protein signaling, and it has been proposed that that is the reason that fentanyl causes more respiratory depression per unit of euphoria when compared to either opiates or methadone type opioids. Likely very few drugs will have an all or nothing bias.Thanks for clarifying!
I'm still trying to figure out what some of what you typed means (I'm ignorant lol) but basically if an opioid causes beta arrestin signalling, would have an effect on it's perceived euphoria? I've read into how it can increase or decrease tolerance, but i'm curious how exactly it might make an opioid more enjoyable.
And yes, definitely the circadian rhythm is what I was thinking.
Would be great if bupe did the reverse & gave me energy during the day when I actually needed it. lol
