Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
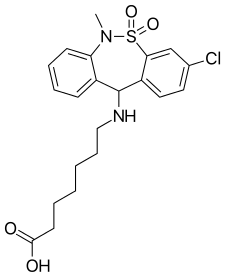
Literature References: Tricyclic compound with psychostimulant, anti-ulcer and anti-emetic properties. Prepn: C. Malen et al., DE 2011806 corresp to US 3758528 (1970, 1973 both to Sci. Union et Cie-Soc. Franc. Rech. Med.). Neuropharmacology study: C. Malen, J. Poignant, Experientia 28, 811 (1972).
Human MOR Ki - 194±70 nM
I am always fascinated when researchers discover a totally unexpected activity in a new medicine. The benzylamine motif is found in quite a few of the more modern opioids from ciramadol to BDPC, from the 3-henyl-3-amino-propionamide derivatives (reference already on the site) to metopholine (and homologues). But you will notice that iin every case, the benzene ring is meta or para substituted? The meta is almost always an -OH therefore the ring is phenolic therefore antagonist activity is possible.... but the para substitution is generally a halogen. I DID find reference to a ciramadol homologue in which the m-OH (aromatic a meta phenol) was swapped for a p-Cl. Sadly the paper was unavailable, but their is certainly a QSAR being built up here.
So, using that basis, the -Cl of tianeptine is para to the benzylamine motif. In this case the amine is secondary, but their are other examples of this and just about the first thing to do if searching to improve MOR Ki is to make that amine tertiary. Of course, the heptanoic acid N-substitution does make the structure the 'magic' 15 methylenes long.
Modelling depression in animals is VERY crude. We don't really understand how it manifests and so we have to derive activity from behavior. Crude AND cruel.
But does the sulfonamide appear in any other opioids? Yes it does. IC26 uses a ethylsulfonyl as bioisostere for methadone's ethyl ketone, the ethylsulfonyl analogue of ketobemidone is known. Data-miners may also note that when fentanyl was being developed, as well as the amide series, Janssen also produced a carbamate ester and a sulfonamide series (I am guessing HE had read of them being used as bioisosteres in opioids). Actually, for people who live in nations where novel RCs are still legal, the carbamate esters of the fentanyl series looks promising.
Finally there is that second aromatic (benzene) ring. It would be interesting to substitute it with, say, a thiophene or furan. There are a stack of 5-membered aromatics but I presume the chemistry of the one's I suggest makes them the logical start point. IF the 2-thienyl homologue (for example) were equipotent (or better) then it's reasonable to suspect where that would overlay fentanyl (for example).
What I THINK happened was that the designers of this medicine began with amineprtine and sought to make it less a specific dopamine releaser and rather something with a broader scope of action (or 'dirtier' as it is termed in pharma development teams). I haven't tried aminpertine but apparently there were 186 doctor's reports in France of patients who had become dependent on the stuff. for it's stimulant properties - no mention of opiate activity. It strongly suggests that the p-Cl making the compound 15 methylenes long is a KEY factor in it's MOR affinity.
But it provides a view to the sheer scope of the 6-(benzylamino)hexanoic acid motif. I suggest that the carboxylic acid moiety might have been chosen because getting such a large alkyl chain through the BBBis rather tricky. I mean, I know THC manages it, but THC is exceptional in many ways.

