I enclose images of tilidine and how it morphs to isonortilidine.
All 3 of them have some mu (opioid) activity, some NMDA antagonist activity and some DRI activity.
fencamfamine relatives lack an ester or reversed ester and so their activity is DRI>NMDA/Mu. When their is an ester (or reversed ester) their is significantly more mu activity, more NMDA activity and retail significant DRI activity.
I recommend that people overlay isonortilidine wirh cypenamine - you will see that they fit exactly.
On unusual feature of tilidine & INT is that it is the secondary amines that are opioids. Tests show that the parent compound (tilidine) has almost no opioid activity or NMDA activity and somewhat less DRI activity.
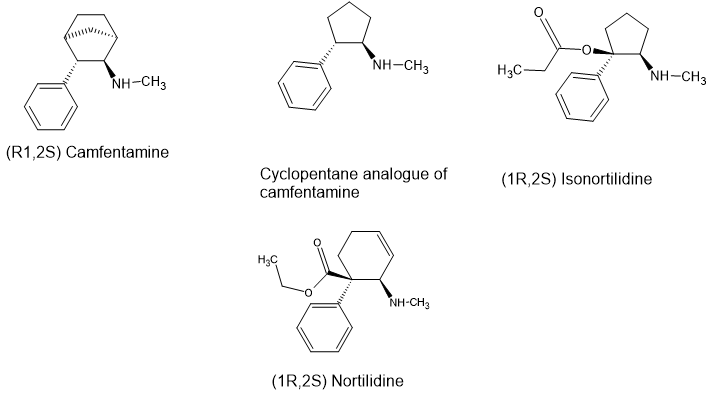
The fact that NMDA activity is mediated by secondary amines suggest that the QSAR is more closely related to ketamine than to PCP. The opioid activity of nortilidine and INT is similar to morphine. The DRI activity is also mediated by the secondary amine although the tertiary amine has some activity.
For those of you with ChemOffice, why not overlay INT and camfentamine - you will see that the overlay is precise.
It seems likely that adding an ester to the benzylic carbon of camfentamine would produce a reasonably potent opioid. After all, it overlays perfectly, compared to tilidine, it's biosteric minimum is not much larger and the bicyclic ring lends to enhanced rigidity.
On may ask how tilidine was discovered. Well, like may things, basic research was carried out using common precursors and using the Diels-Alder reaction (in the way etorphine was discovered).
All 3 of them have some mu (opioid) activity, some NMDA antagonist activity and some DRI activity.
fencamfamine relatives lack an ester or reversed ester and so their activity is DRI>NMDA/Mu. When their is an ester (or reversed ester) their is significantly more mu activity, more NMDA activity and retail significant DRI activity.
I recommend that people overlay isonortilidine wirh cypenamine - you will see that they fit exactly.
On unusual feature of tilidine & INT is that it is the secondary amines that are opioids. Tests show that the parent compound (tilidine) has almost no opioid activity or NMDA activity and somewhat less DRI activity.

The fact that NMDA activity is mediated by secondary amines suggest that the QSAR is more closely related to ketamine than to PCP. The opioid activity of nortilidine and INT is similar to morphine. The DRI activity is also mediated by the secondary amine although the tertiary amine has some activity.
For those of you with ChemOffice, why not overlay INT and camfentamine - you will see that the overlay is precise.
It seems likely that adding an ester to the benzylic carbon of camfentamine would produce a reasonably potent opioid. After all, it overlays perfectly, compared to tilidine, it's biosteric minimum is not much larger and the bicyclic ring lends to enhanced rigidity.
On may ask how tilidine was discovered. Well, like may things, basic research was carried out using common precursors and using the Diels-Alder reaction (in the way etorphine was discovered).



