haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826
The 2 & 3 carbon phenethylamines have been studied. Shulgin amongst others have made probably thousands of individual compounds (and in the most part taste them).
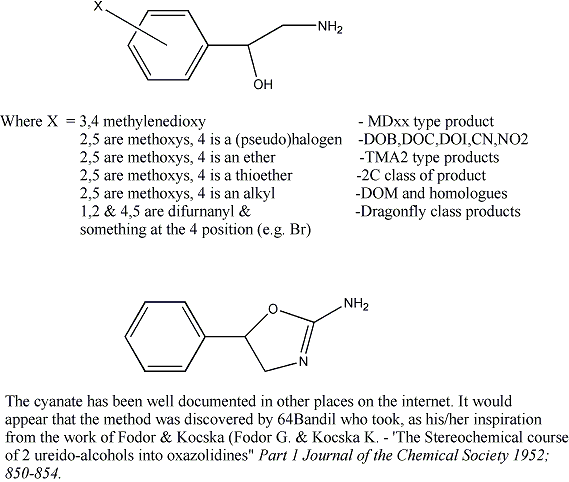
But what of the aminorex series? The 4-methyl ones are somewhat out because the starting compound is is substituted phenyl propanol amine which is illegal in most places. The 2 carbon one, however, falls outside the phenylethyl scheduling since it has an alpha -OH. For example, what is the legal status of 1(3,4 methylenedioxy) phenyl, 1 hydroxyl 3 ethylamine?
The old cyanogen bromide is out and the new cyanate route is in. I'm just wondering if we have the possibility of an entirely new series to look into. U4euH is sort of MDxx like in any case, so the methylenedioxy analog might be the best thing since bread (even better than sliced bread, you understand).
But what of the aminorex series? The 4-methyl ones are somewhat out because the starting compound is is substituted phenyl propanol amine which is illegal in most places. The 2 carbon one, however, falls outside the phenylethyl scheduling since it has an alpha -OH. For example, what is the legal status of 1(3,4 methylenedioxy) phenyl, 1 hydroxyl 3 ethylamine?
The old cyanogen bromide is out and the new cyanate route is in. I'm just wondering if we have the possibility of an entirely new series to look into. U4euH is sort of MDxx like in any case, so the methylenedioxy analog might be the best thing since bread (even better than sliced bread, you understand).