-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Sodium under the microscope
- Thread starter lemonman
- Start date
doxylamine
Greenlighter
- Joined
- Aug 8, 2010
- Messages
- 47
Individual atoms can only be seen with the most powerful of microscopes such as a Scanning Tunneling Microscope and even then they only appear as round figures, regardless of what atom it is. See image below.

As for the actual structure of a sodium atom, see image below.
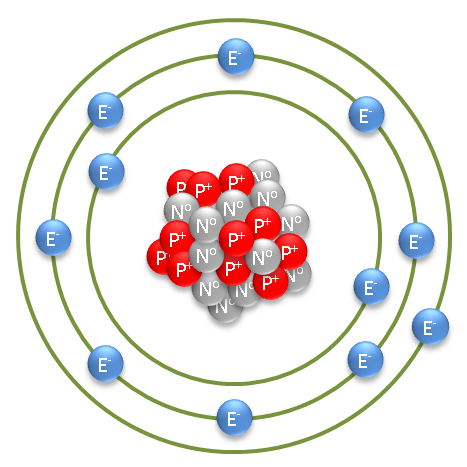
P+ and N0 represent the protons and neutrons, respectively, within the nucleus and E- represents electrons.
The green concentric circles represent the electron orbitals. They are kind of akin to lanes in a racetrack, whereby the negatively charged electrons orbit the positively charged nucleus.

As for the actual structure of a sodium atom, see image below.

P+ and N0 represent the protons and neutrons, respectively, within the nucleus and E- represents electrons.
The green concentric circles represent the electron orbitals. They are kind of akin to lanes in a racetrack, whereby the negatively charged electrons orbit the positively charged nucleus.
Last edited:
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Sodium or neurotransmitter atoms and molecules respectively, outside a living system of course could be imaged under an electron microscope.
If this isn't a homework Q, and you just haven't seen it before, here is a pic. Its of a block of lab-grade elemental sodium, a very light-weight, low density metal that when freshly cut has a brilliant shiny lustre but rapidly oxidizes in air to the duller grey you see on this. It came in a double hermetically sealed bag-in-a-bag, the metal coated in some kind of paraffin oil type inert to help preserve it, then evacuated and heatsealed in an inert environment, the inner bag thus created fitting tight to the block of sodium
The outer bag contains this, in an inert atmosphere, hermetically sealed.
Its so soft, it can be cut with a blunt butterknife, or even made to deform in a plastic manner by squeezing between the fingers. The chunk you see here has the characteristic oxidation color, a duller grey on the outside rather than the brilliant shine of Na freshly melted and popped into high-boiling dry inert naphtha type non-aromatic solvent, or freshly cut pieces from a block (sorry, but I'm not about to take pictures of that, since I'd have to open it, and I am saving this until I need the full block for something so I can't show you the shine, since it'd leave my Na with a far lesser shelf life.) Nothing personal, but I did have to pay for it, and I'm not going to have any of it consumed uselessly
Total weight of the block-100 grams, which makes for a square chunk a tiny bit smaller to about the same as my tight-clenched fist.
So soft, it deforms in a plastic-type way with just the pressure exerted when squeezed between thumb and forefinger.
https://s17.postimg.org/6q6f40anz/image.jpg
If this isn't a homework Q, and you just haven't seen it before, here is a pic. Its of a block of lab-grade elemental sodium, a very light-weight, low density metal that when freshly cut has a brilliant shiny lustre but rapidly oxidizes in air to the duller grey you see on this. It came in a double hermetically sealed bag-in-a-bag, the metal coated in some kind of paraffin oil type inert to help preserve it, then evacuated and heatsealed in an inert environment, the inner bag thus created fitting tight to the block of sodium
The outer bag contains this, in an inert atmosphere, hermetically sealed.
Its so soft, it can be cut with a blunt butterknife, or even made to deform in a plastic manner by squeezing between the fingers. The chunk you see here has the characteristic oxidation color, a duller grey on the outside rather than the brilliant shine of Na freshly melted and popped into high-boiling dry inert naphtha type non-aromatic solvent, or freshly cut pieces from a block (sorry, but I'm not about to take pictures of that, since I'd have to open it, and I am saving this until I need the full block for something so I can't show you the shine, since it'd leave my Na with a far lesser shelf life.) Nothing personal, but I did have to pay for it, and I'm not going to have any of it consumed uselessly
Total weight of the block-100 grams, which makes for a square chunk a tiny bit smaller to about the same as my tight-clenched fist.
So soft, it deforms in a plastic-type way with just the pressure exerted when squeezed between thumb and forefinger.
https://s17.postimg.org/6q6f40anz/image.jpg
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
That block of sodium has been quite well oxidised - seems to be coated in sodium oxide/hydroxides by now. Sodium really should be stored under argon, kerosine or under mineral oil.
It's also worth remembering that sodium(0) (the reactive, reducing, alkali metal) and sodium(1+) (the cation present in all aqueous environments and biological systems) are pretty different things that act quite differently.
Whuts in the reagent jars, L_C? something [dimethylamino]ethane? And isopropylamine?
It's also worth remembering that sodium(0) (the reactive, reducing, alkali metal) and sodium(1+) (the cation present in all aqueous environments and biological systems) are pretty different things that act quite differently.
Whuts in the reagent jars, L_C? something [dimethylamino]ethane? And isopropylamine?
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Its diisopropylaminoethanol, and some diphenylacetic acid. Got my eyes on some Hunig's base, given the non-nucleophilic nature of diisopropylethylamine, then conversion to the bromide or iodide, tosylate etc. and hydrogenation ought to do the trick, no? Especially using a good leaving group like tosylate, brosylate or nosylate. As for the diphenylacetic acid then I'll leave what thats for to the imagination. And the ones behind it are a tub of sodium dichromate, and bottles of n-butyric anhydride (if ever a reagent was a real pleasure to work with....then you'll LOVE butryic anhydride  ), propionyl chloride, benzoyl chloride and trans-cinnamyl chloride. The bottles and containers wrapped in an outer layer are 2-pyrollidone (been thinking maybe some racetams, phenylpiracetam super cheap, would be rather neat, from what I've heard of it. Had good results with pramiracetam, but so far haven't tried phenylpiracetam. Phenylaniracetam, phenylpramiracetam etc. might be interesting candidates to explore) and I think one of them might be borohydride. The one with the darker inside and the full wrapping tied on is 2-pyrollidone, and the greyish thing behind the sodium is a can of NaBH4, or rather, the metal can the bottle came in, with the bottle sealed inside, the outside purged with argon and a covering stuck over the top, although my borohydride should have a pretty good shelf life, as its in the form of convenient 1g-per-piece tablets rather than powder. (not that those would be 'tablets' anybody would want to swallow xD )
), propionyl chloride, benzoyl chloride and trans-cinnamyl chloride. The bottles and containers wrapped in an outer layer are 2-pyrollidone (been thinking maybe some racetams, phenylpiracetam super cheap, would be rather neat, from what I've heard of it. Had good results with pramiracetam, but so far haven't tried phenylpiracetam. Phenylaniracetam, phenylpramiracetam etc. might be interesting candidates to explore) and I think one of them might be borohydride. The one with the darker inside and the full wrapping tied on is 2-pyrollidone, and the greyish thing behind the sodium is a can of NaBH4, or rather, the metal can the bottle came in, with the bottle sealed inside, the outside purged with argon and a covering stuck over the top, although my borohydride should have a pretty good shelf life, as its in the form of convenient 1g-per-piece tablets rather than powder. (not that those would be 'tablets' anybody would want to swallow xD )
And its covered in mineral oil, its just surface oxidation, the actual inner lining has been vac/heatsealed by the manufacturer, no change observed since it first came, and if one squeezes it etc. then there is clearly a slightly thick fluid over the surface, doubtless some sort of kerosene, mineral spirits etc. And its in an outer bag full of some inert gas. And getting (and keeping) the surface of a block of alkali metal pristine shiny is...well it takes melting and ampouling under inert atmosphere, skimming any oxide layer off and then sealing it under inert gas, I don't think I've ever seen a lab-supply of sodium metal that doesn't come in sealed vials that doesn't have some oxidation on the surface. And I have had that particular block a year or two, and it still looks just as it did when it came in the post (I've often thought...I REALLY wouldn't like to be a postie, out in filthy weather and the freezing cold come what may. But being MY postman....I'd be looking for a new job )
)
I don't expect it to stay fresh and shiny very much when supplied, even in oil etc. there will be a degree of surface oxidation. And since I first obtained it and until now, there hasn't been any visible.
And, you missed the other oxidation state of the alkali metals. -1. The alkalides. Kinda neat IMO, even forming a sodium hydride where the negative charge is on the Na atom. Aka 'inverse sodium hydride', the alkali metal behaving as the anion whilst the H bears the positive charge, prepared using cryptand ligands followed by a metathesis rxn to give Na-H+, that have some some similarity to electrides. Always thought it might be fun to attempt the synthesis. And using dimethyl ether as a solvent for extraction and crystallization, how convenient is that? since MeOMe just boils off as if it were hardly there to begin with, especially if it were done with vacuum after purging the system with inert gas, quickest solvent crystallization EVER.
(still got to get the last couple of pieces of my rotavap sent)
Finally getting the pork off my arse too it seems. Started a case against them and planning to take it all the way (and after what they have done lately, IMO they are going to be ankle deep in dogshit after I've finished. From the neck up
 ), propionyl chloride, benzoyl chloride and trans-cinnamyl chloride. The bottles and containers wrapped in an outer layer are 2-pyrollidone (been thinking maybe some racetams, phenylpiracetam super cheap, would be rather neat, from what I've heard of it. Had good results with pramiracetam, but so far haven't tried phenylpiracetam. Phenylaniracetam, phenylpramiracetam etc. might be interesting candidates to explore) and I think one of them might be borohydride. The one with the darker inside and the full wrapping tied on is 2-pyrollidone, and the greyish thing behind the sodium is a can of NaBH4, or rather, the metal can the bottle came in, with the bottle sealed inside, the outside purged with argon and a covering stuck over the top, although my borohydride should have a pretty good shelf life, as its in the form of convenient 1g-per-piece tablets rather than powder. (not that those would be 'tablets' anybody would want to swallow xD )
), propionyl chloride, benzoyl chloride and trans-cinnamyl chloride. The bottles and containers wrapped in an outer layer are 2-pyrollidone (been thinking maybe some racetams, phenylpiracetam super cheap, would be rather neat, from what I've heard of it. Had good results with pramiracetam, but so far haven't tried phenylpiracetam. Phenylaniracetam, phenylpramiracetam etc. might be interesting candidates to explore) and I think one of them might be borohydride. The one with the darker inside and the full wrapping tied on is 2-pyrollidone, and the greyish thing behind the sodium is a can of NaBH4, or rather, the metal can the bottle came in, with the bottle sealed inside, the outside purged with argon and a covering stuck over the top, although my borohydride should have a pretty good shelf life, as its in the form of convenient 1g-per-piece tablets rather than powder. (not that those would be 'tablets' anybody would want to swallow xD )And its covered in mineral oil, its just surface oxidation, the actual inner lining has been vac/heatsealed by the manufacturer, no change observed since it first came, and if one squeezes it etc. then there is clearly a slightly thick fluid over the surface, doubtless some sort of kerosene, mineral spirits etc. And its in an outer bag full of some inert gas. And getting (and keeping) the surface of a block of alkali metal pristine shiny is...well it takes melting and ampouling under inert atmosphere, skimming any oxide layer off and then sealing it under inert gas, I don't think I've ever seen a lab-supply of sodium metal that doesn't come in sealed vials that doesn't have some oxidation on the surface. And I have had that particular block a year or two, and it still looks just as it did when it came in the post (I've often thought...I REALLY wouldn't like to be a postie, out in filthy weather and the freezing cold come what may. But being MY postman....I'd be looking for a new job
 )
)I don't expect it to stay fresh and shiny very much when supplied, even in oil etc. there will be a degree of surface oxidation. And since I first obtained it and until now, there hasn't been any visible.
And, you missed the other oxidation state of the alkali metals. -1. The alkalides. Kinda neat IMO, even forming a sodium hydride where the negative charge is on the Na atom. Aka 'inverse sodium hydride', the alkali metal behaving as the anion whilst the H bears the positive charge, prepared using cryptand ligands followed by a metathesis rxn to give Na-H+, that have some some similarity to electrides. Always thought it might be fun to attempt the synthesis. And using dimethyl ether as a solvent for extraction and crystallization, how convenient is that? since MeOMe just boils off as if it were hardly there to begin with, especially if it were done with vacuum after purging the system with inert gas, quickest solvent crystallization EVER.
(still got to get the last couple of pieces of my rotavap sent)
Finally getting the pork off my arse too it seems. Started a case against them and planning to take it all the way (and after what they have done lately, IMO they are going to be ankle deep in dogshit after I've finished. From the neck up

Last edited:
