Hey there BL, its been a while with my studies and all haha.
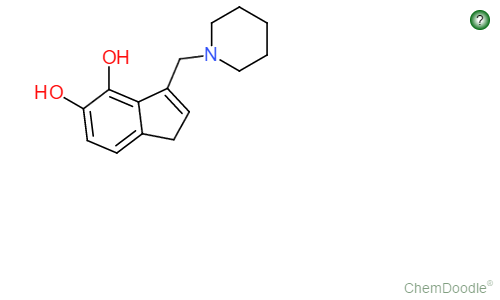
So I realize my title is actually not correct and this suggestion wouldnt be modifying the backbone itself, but I digress....I love doodling random structures in class when the subject matter is stuff I already know and one of the theoretical drugs I drew up the following structure (http://imgur.com/LgVT6wE)
The way it is drawn, the two hydroxys at 4 and 5 can be substituted with anything imaginable (MeO, Aco, Indene, etc.) or not be included at all, like a piperidine version of dmt, but they are there just to show the variable sites of interest.
The real interesting thing about this molecule is its combination of being a piperidine and also having the indole based backbone of a tryptamine.
Just out of absolute curiosity, does this chemical seem like it would be stable? I have no clue how to go about making such a drug but it seems like it is almost certainly psychoactive, and with such a spread of hydrophilicity and lipophilicty, I would bet its absurdly potent. The only real issue would be steric hindrance I imagine, but what are some other peoples thoughts? This is purely a hypothetical discussion and I cant stress that enough. There is just as equal likelihood that something like this would be dangerous, or not work at all but for the sake of science I would love to hear bluelights take on this.
Much love and I look forward to responses!
So I realize my title is actually not correct and this suggestion wouldnt be modifying the backbone itself, but I digress....I love doodling random structures in class when the subject matter is stuff I already know and one of the theoretical drugs I drew up the following structure (http://imgur.com/LgVT6wE)
The way it is drawn, the two hydroxys at 4 and 5 can be substituted with anything imaginable (MeO, Aco, Indene, etc.) or not be included at all, like a piperidine version of dmt, but they are there just to show the variable sites of interest.
The real interesting thing about this molecule is its combination of being a piperidine and also having the indole based backbone of a tryptamine.
Just out of absolute curiosity, does this chemical seem like it would be stable? I have no clue how to go about making such a drug but it seems like it is almost certainly psychoactive, and with such a spread of hydrophilicity and lipophilicty, I would bet its absurdly potent. The only real issue would be steric hindrance I imagine, but what are some other peoples thoughts? This is purely a hypothetical discussion and I cant stress that enough. There is just as equal likelihood that something like this would be dangerous, or not work at all but for the sake of science I would love to hear bluelights take on this.
Much love and I look forward to responses!

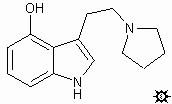
 lol.
lol.