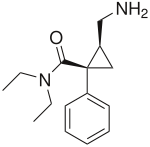
This is milnacipran... Somehow I have a feeling that if you dialkylated that primary amine you would end up with an antagonist or partial agonist...
N,Ndimethyl milnacipran
has been synthesized and studied a couple of times in recent years, in SAR studies of milnacipran analogues. I can't get my banana-republic university account to work right now but if somebody can look through the following, I'd appreciate it:
Studies on the SAR and pharmacophore of milnacipran derivatives as monoamine transporter inhibitors.
As the title implies, the focus is on reuptake inhibition and I doubt it or other milnacipran analogues have been assessed for opiod receptor affinity. I assume this will be looked into in the next few years, since the 1R, 2S enantiomer of milnacipran may flood the US SNRI market soon, as levomilnacipran:
The racemic mixture's trade name is Savella here and is FDA approved for certain neuropathies. The SNRI market is so saturated right now and there are more efficacious options than racemic milnacipran. But the company that's developing the 1R, 2S enantiomer is the same that thrived in the-immensely saturated SSRI market a few years ago with escitalopram--which I think remains the most potent FDA-approved SERT inhibitor. With the citaloprams, it turned out that the R-enantiomer was allosterically inhibiting the S-enantiomer. Once the mixture was resolved, SERT inhibition increased 20% (I think). Mediocre SSRI became top-of-the-line SSRI (at least to investor's ears; many patients prefer sertraline).
The same may be true for 1R, 2S milnacipran (I haven't done the research yet but the developer still has street-cred for escitalopram). I forget what the proposed mechanism is for SNRI analgesia, and I think I remember it somehow indirectly entails opiate receptor pathways (I'm not saying that SNRIs are MOR ligands). It'll be interesting to see if any milnacipran analogue or derivative that satisfies the morphine rule turns out to bind to opiate receptors without sacrificing its SNRI activity.