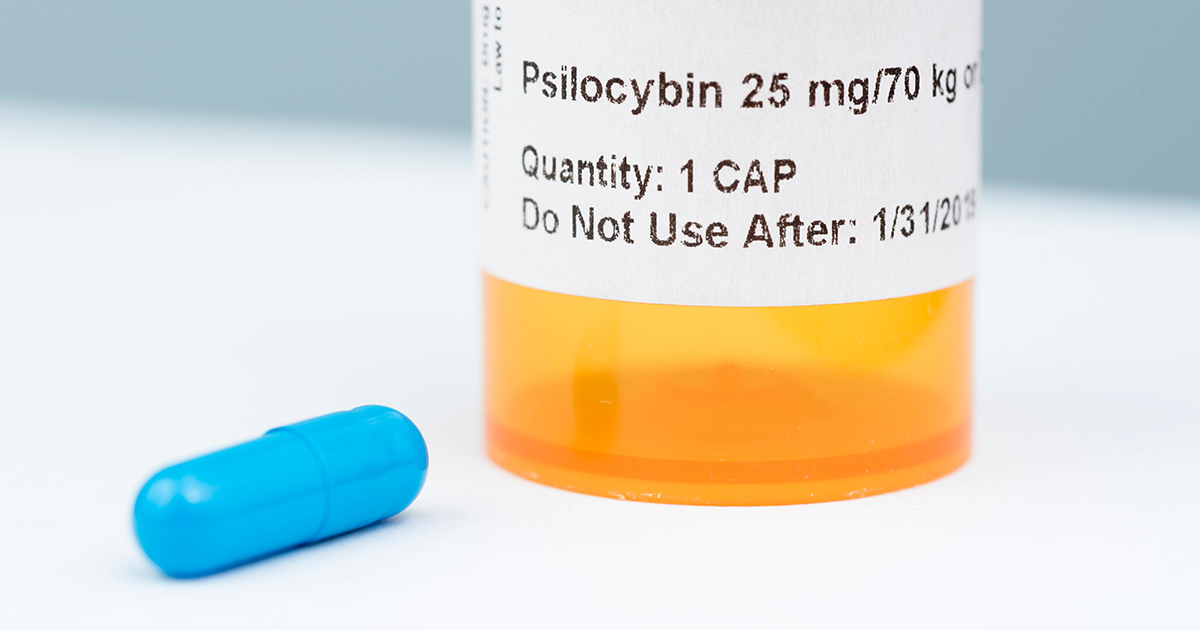mr peabody
Bluelight Crew

Imperial College London
Your brain on LSD: A guide*
by David Nutt | Science Focus | 28 Jan 2021
David Nutt is a professor of neuropsychopharmacology in Imperial College London. In this extract from his book, Drugs without the hot air, he explains how psychedelic drugs can be used for treating a variety of mental health conditions, and can even help with problem-solving.
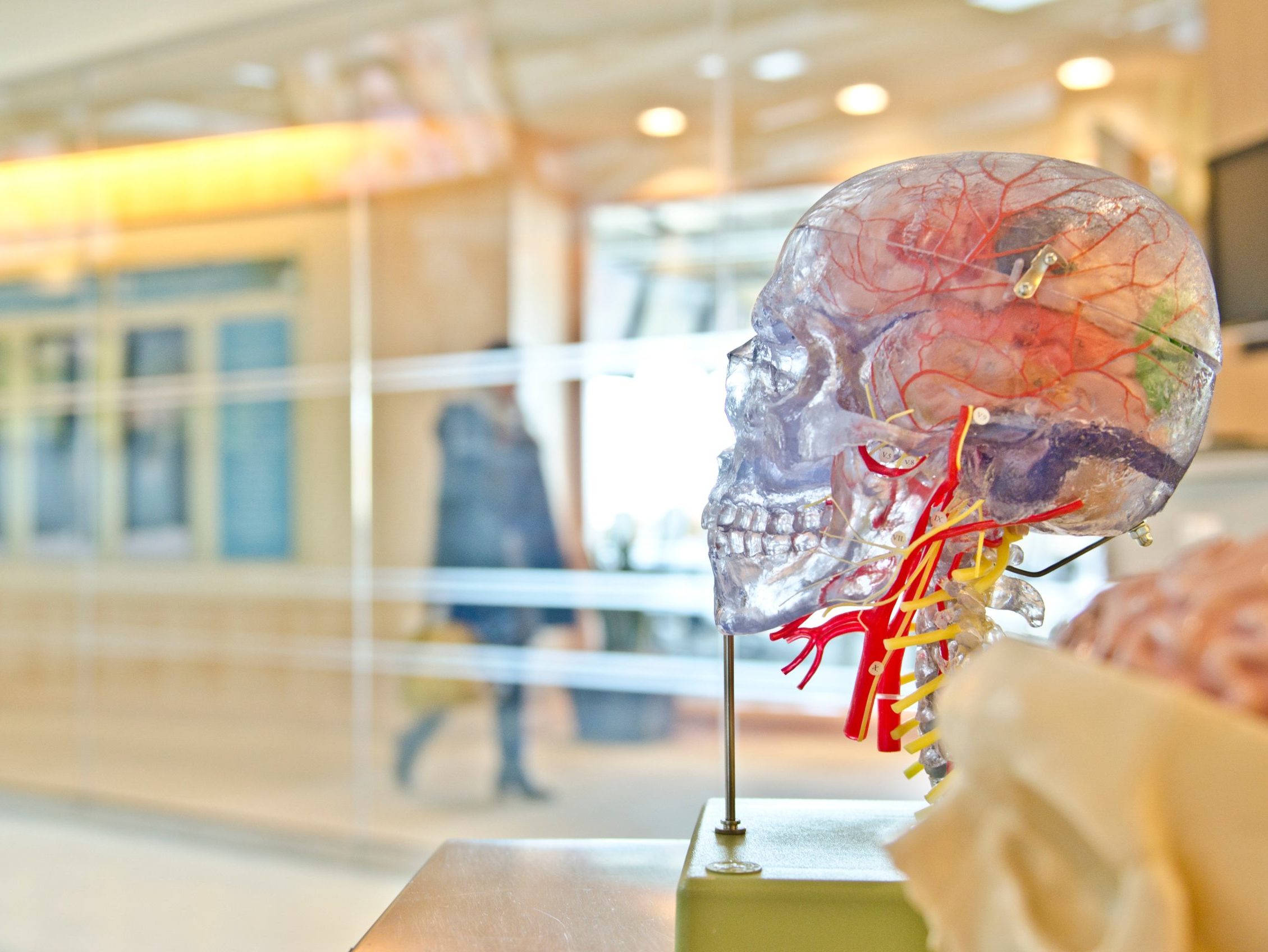
Since the first edition of this book there has been a veritable explosion of research on psychedelics, with brain-imaging studies on psilocybin, ayahuasca and even LSD, and several landmark clinical trials. Many of these have come from the team led by Robin Carhart-Harris in my unit.
In collaboration with the Beckley Foundation we have conducted three imaging studies with psilocybin, two using fMRI, one using MEG (mag- netoencephalography), and the first-ever imaging study of LSD. Other groups have conducted similar studies – in Barcelona (with ayahuasca) and in Zurich (with psilocybin and LSD).
These have confirmed the first findings with psilocybin reported earlier in this chapter [that psilocybin administered in a controlled environment can help to treat depression].
Psychedelics produce a profoundly altered state of consciousness which can be explained by a marked loosening of the normal constraints on brain functioning. In the psychedelic state the brain is less constrained, more flexible and more interconnected.
This explains the emergence of complex hallucinations and altered feelings such as being at one with the Universe. To paraphrase the words of William Blake, which were used by Aldous Huxley to explain his psychedelic experiences: The doors of perception are opened, so man can see everything as it is – infinite.
Along with these new insights into the neuroscience of psychedelics has come the use of psilocybin to help dying people be reconciled to their imminent death, or help patients with major psychiatric problems (such as resistant depression) come to terms with their condition.
There have also been pilot studies on using psilocybin to treat smoking and alcohol dependence, and above we referred to our team’s 2016 controlled trial of psilocybin for resistant depression. We are now repeating this study but this time comparing the effect of psilocybin with that of escitalopram (a strong SSRI). This study funded by the Alexander Mosley Charitable Trust will allow us to compare whether the two treatments are the same in terms of their brain mechanisms or, as we suspect, rather different.
Also at the end of 2016 the two largest placebo-controlled clinical trials of psilocybin ever conducted were reported by Roland Griffiths of Johns Hopkins and Stephen Ross of New York University, both in the US. The designs were similar so they each provided a form of validation for each other.
Both showed a significant benefit of psilocybin – again at a single 25 mg dose – to reduce the anxiety and depression that people who are facing death from untreatable medical conditions usually experience.
These results were well received, with several past-presidents of the American Psychiatric Association writing in favour of more research with psychedelics. On the basis of this enthusiasm and the efficacy demonstrated in two independent and sizeable studies, there is now a plan to push to have psilocybin licensed for treating depression, and this approach has been endorsed as viable by the FDA (Food and Drug Administration) in the US.
What it will take is a major multi-centre study and this is now underway, being conducted by COMPASS Pathways using the same 25 mg oral dose that we used. The results should be available in 2021 and, if positive, psilocybin might be allowed as a medicine a couple of years after that.

Should scientists take LSD?
One line of enquiry which was being studied before LSD was made illegal was its use in problem-solving. This may seem surprising because most people find it hard to focus when tripping, and it’s certainly true that people taking LSD don’t perform particularly well on standard psychological tests.
As psychologist Arthur Kleps explained in 1966, “if I were to give you an IQ test and during the administration one of the walls of the room opened up giving you a vision of the blazing glories of the central galactic suns, and at the same time your childhood began to unreel before your inner eye like a three-dimensional colour movie, you would not do well on the test.”
However, psychedelics can induce creativity, by lowering the psychological defences around “getting something wrong” and helping to see problems from new angles. With the right set and setting, this can be directed towards familiar problems, sometimes with spectacular results.
This approach seems to work best when the problem is one that has been considered many times while sober, and the setting includes notes, lots of paper and pens to record your thoughts, and a guide to help you through the initial disorientation and remind you of what your aim is.
Although most people who have tried psychedelics might find it hard to believe that it would be possible to concentrate on a task during a trip, that’s probably because they’ve taken them in circumstances where they were easily distracted – and they’ve probably also been surrounded by other people on drugs at the same time.
In a calm setting it’s much easier to focus, and in fact people on LSD are so suggestible that if they have a guide who tells them that they will have no difficulty concentrating, this will probably be the case.
Taking LSD in this way has been known to deliver moments of inspiration to designers, architects and engineers. There are many stories about near-perfect technical designs which have suddenly become obvious while contemplating the problem on the drug.
In LSD – the Problem Solving Psychedelic, written in 1967, the authors recount several examples of this. One was a furniture designer who completed a chair design while on LSD which was successfully modelled into a functional dining chair with no substantial changes from the original concept. (These pieces of furniture are extremely difficult to create and the designer was used to new chairs taking two months and ten trial models to complete.)
Another example was an engineer who worked in Naval Research and had been trying to design a special detection device for five years without success. Within minutes of contemplating the problem on LSD he had found the solution and the device was then patented and used by the US Navy.
A third example was an architect who took the drug to help him design a shopping centre and was able to visualise it in its entirety: “Suddenly I saw the finished project. I did some quick calculations… it would fit on the property and not only that… it would meet the cost and income requirements… I began to draw… my senses could not keep up with my images.” The image stayed with him just as sharply after the drug experience had ended, and his design was accepted and constructed.
Even in the less-obviously creative fields of hard science, LSD can be profoundly beneficial. In fact, it played a role in the two biggest discoveries in biology of the 20th Century.
Francis Crick, who discovered the double helix structure of DNA with James Watson, and Kary Mullis, who invented the polymerase chain reaction (PCR), had both taken the drug, and attributed some of their understanding and insights to it.
Mullis has gone so far as to say: “would I have invented PCR if I hadn’t taken LSD? I seriously doubt it… having taken LSD I could sit on a DNA molecule and watch the polymers go by. I learnt that partly on psychedelic drugs.”
Both Crick and Mullis received Nobel prizes for their work. Now that LSD is illegal few scientists would dare to use it, and even fewer would own up to taking it. Perhaps scientific progress in many areas has been hindered by this state of affairs!
*From the article here :

 www.sciencefocus.com
www.sciencefocus.com
As psychologist Arthur Kleps explained in 1966, “if I were to give you an IQ test and during the administration one of the walls of the room opened up giving you a vision of the blazing glories of the central galactic suns, and at the same time your childhood began to unreel before your inner eye like a three-dimensional colour movie, you would not do well on the test.”
However, psychedelics can induce creativity, by lowering the psychological defences around “getting something wrong” and helping to see problems from new angles. With the right set and setting, this can be directed towards familiar problems, sometimes with spectacular results.
This approach seems to work best when the problem is one that has been considered many times while sober, and the setting includes notes, lots of paper and pens to record your thoughts, and a guide to help you through the initial disorientation and remind you of what your aim is.
Although most people who have tried psychedelics might find it hard to believe that it would be possible to concentrate on a task during a trip, that’s probably because they’ve taken them in circumstances where they were easily distracted – and they’ve probably also been surrounded by other people on drugs at the same time.
In a calm setting it’s much easier to focus, and in fact people on LSD are so suggestible that if they have a guide who tells them that they will have no difficulty concentrating, this will probably be the case.
Taking LSD in this way has been known to deliver moments of inspiration to designers, architects and engineers. There are many stories about near-perfect technical designs which have suddenly become obvious while contemplating the problem on the drug.
In LSD – the Problem Solving Psychedelic, written in 1967, the authors recount several examples of this. One was a furniture designer who completed a chair design while on LSD which was successfully modelled into a functional dining chair with no substantial changes from the original concept. (These pieces of furniture are extremely difficult to create and the designer was used to new chairs taking two months and ten trial models to complete.)
Another example was an engineer who worked in Naval Research and had been trying to design a special detection device for five years without success. Within minutes of contemplating the problem on LSD he had found the solution and the device was then patented and used by the US Navy.
A third example was an architect who took the drug to help him design a shopping centre and was able to visualise it in its entirety: “Suddenly I saw the finished project. I did some quick calculations… it would fit on the property and not only that… it would meet the cost and income requirements… I began to draw… my senses could not keep up with my images.” The image stayed with him just as sharply after the drug experience had ended, and his design was accepted and constructed.
Even in the less-obviously creative fields of hard science, LSD can be profoundly beneficial. In fact, it played a role in the two biggest discoveries in biology of the 20th Century.
Francis Crick, who discovered the double helix structure of DNA with James Watson, and Kary Mullis, who invented the polymerase chain reaction (PCR), had both taken the drug, and attributed some of their understanding and insights to it.
Mullis has gone so far as to say: “would I have invented PCR if I hadn’t taken LSD? I seriously doubt it… having taken LSD I could sit on a DNA molecule and watch the polymers go by. I learnt that partly on psychedelic drugs.”
Both Crick and Mullis received Nobel prizes for their work. Now that LSD is illegal few scientists would dare to use it, and even fewer would own up to taking it. Perhaps scientific progress in many areas has been hindered by this state of affairs!
*From the article here :

Your brain on LSD: a guide through the most mind-blowing psychedelics research
In an extract of his acclaimed book Drugs without the hot air, David Nutt explains recent scientific discoveries – and how substances like LSD have influenced creative breakthroughs.
Last edited: