-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
replacing carbon with silicon
- Thread starter black53
- Start date
Have any idea how unstable we're talking about? Goes bad in a few days or half life measured in minutes?
And I do wonder how it would behave in man and would the amphetamine like drug of silicone based life look similar or totally different? Shame science won't be able to answer lots of those in a long time.
And I do wonder how it would behave in man and would the amphetamine like drug of silicone based life look similar or totally different? Shame science won't be able to answer lots of those in a long time.
Have any idea how unstable we're talking about? Goes bad in a few days or half life measured in minutes?
And I do wonder how it would behave in man and would the amphetamine like drug of silicone based life look similar or totally different? Shame science won't be able to answer lots of those in a long time.
Have lives measured in microseconds or less (nanoseconds)...
In case of hexasilabenzene ring, it cannot even be formed unless all H is heavily substituted with steric groups much larger than t-butyl (eg, bis-(trimethylsilyl)methyl, or triisopropylphenyl) on every every single H atom.
(Or it burst into flame, then deposit silica nanoparticles around in some microseconds)
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
The molecule you drew couldn't be synthesized. You would be lucky if you could get 2 or 3 silicon atoms connected before it fell apart to silica (sand). Even if you did synthesize it it would need to be stored under vacuum and with no water around. Silicon doesn't form stable double bonds or aromatics, and the Si-H bond is relatively weak compared to C-H bonds, meaning that silanes like to give up their hydrogens. As a result most silicon atoms in drugs don't have hydrogens attached.
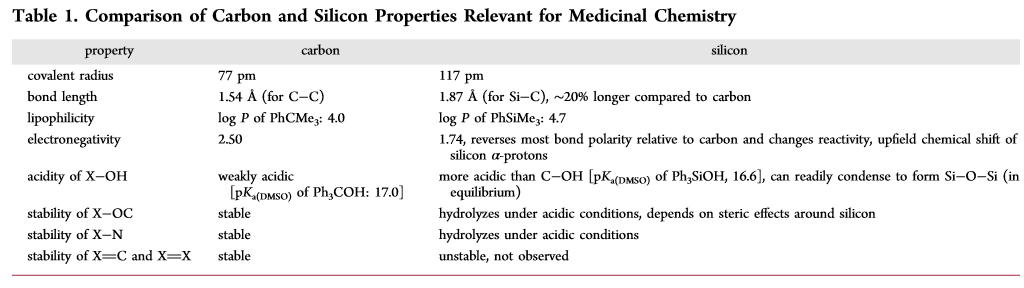
Refs to read:
Organosilicon molecules with medical applications
Pipeline: Odd Elements In Drugs: Silicon
Pipeline: Silicon In Drug Molecules, Not Quite There Yet

Refs to read:
Organosilicon molecules with medical applications
Pipeline: Odd Elements In Drugs: Silicon
Pipeline: Silicon In Drug Molecules, Not Quite There Yet
Last edited:
mad_scientist
Bluelighter
- Joined
- Apr 20, 2006
- Messages
- 619
There are examples of carbon being replaced by silicon in psychoactive drugs and retaining activity (at least in mice), but this can only be done where you have a quaternary carbon atom attached to four other carbon atoms with single bonds. There are relatively few recreational drugs that contain this substructure, many opioids do but generally the structures are too complex to make the silicon version. Still an interesting idea though...
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
I wonder if there is silicone based life out there how those organisms deal with it.
I'm imagining hundreds of sand monsters rising up from the desert, moaning 'H' as they shuffle around, losing arms, legs and heads in the process.
Roger&Me
Bluelighter
- Joined
- Dec 8, 2004
- Messages
- 23,526
Can you think of any Si based molecule that would be stable and perhaps be psychoactive?
stable? sure, glass and etc. psychoactive? no, not really.
i guess you might be able to envision some kind of substituted siloxane polymer that could be engineered to interact with certain proteins, but i've never actually seen anything like that
Roger&Me
Bluelighter
- Joined
- Dec 8, 2004
- Messages
- 23,526
Heh, "glass" not psychoactive..
lol hence why i said "pyschoactive? no"
Glass is psychoactive...lol hence why i said "pyschoactive? no"
1) stare in randomly convex and concave mirror and lens, you will get nausious
2) step on sharp glass accidentally, and you get a sharp rush
3) etc
2) step on sharp glass accidentally, and you get a sharp rush
3) etc
izo
Bluelighter
- Joined
- Mar 22, 2006
- Messages
- 4,165
There are examples of carbon being replaced by silicon in psychoactive drugs and retaining activity (at least in mice), but this can only be done where you have a quaternary carbon atom attached to four other carbon atoms with single bonds. There are relatively few recreational drugs that contain this substructure, many opioids do but generally the structures are too complex to make the silicon version. Still an interesting idea though...
i only know of the following:
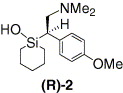
(R)-Sila-venlafaxine: A selective noradrenaline reuptake inhibitor for the treatment of emesis
Sila-substitution of drugs (the carbon/silicon switch) is a concept that is being successfully used for the development of new chemical entities. The …
nepalnt21
Bluelighter
- Joined
- Nov 3, 2016
- Messages
- 2,157
yup, specially if you fill it with aromatic herbsI hear if you smoke it it can be quite good too.
