izo
Bluelighter
MT-45
this was not bad at all at 200mg, a solid opioid, as good as tilidine easily.
N&PD Moderators: Skorpio | someguyontheinternet
MT-45
As far as I can see, the SAR of opioids is straight out of Alice through the looking glass, so many different compound exhibit opioid activity.
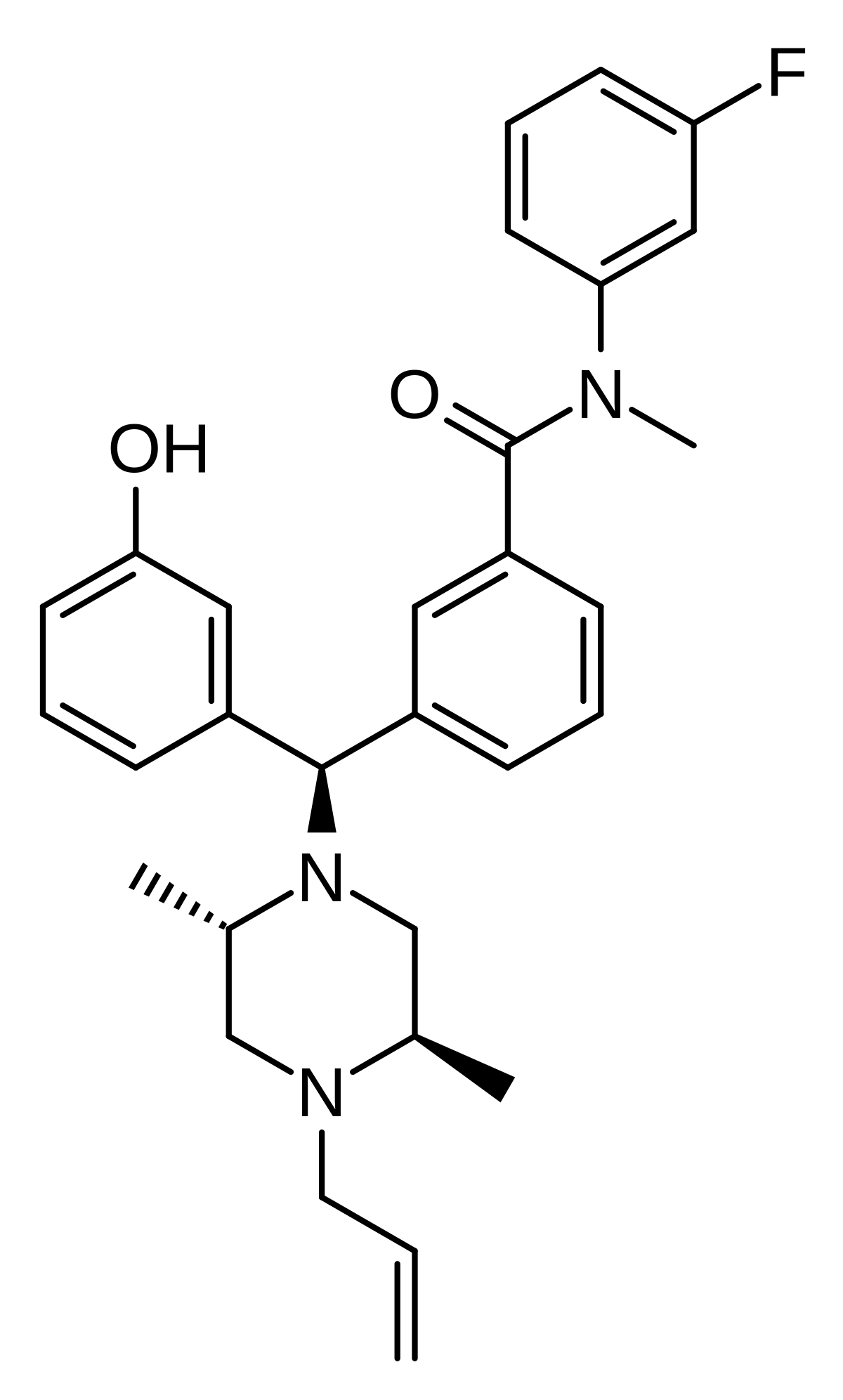
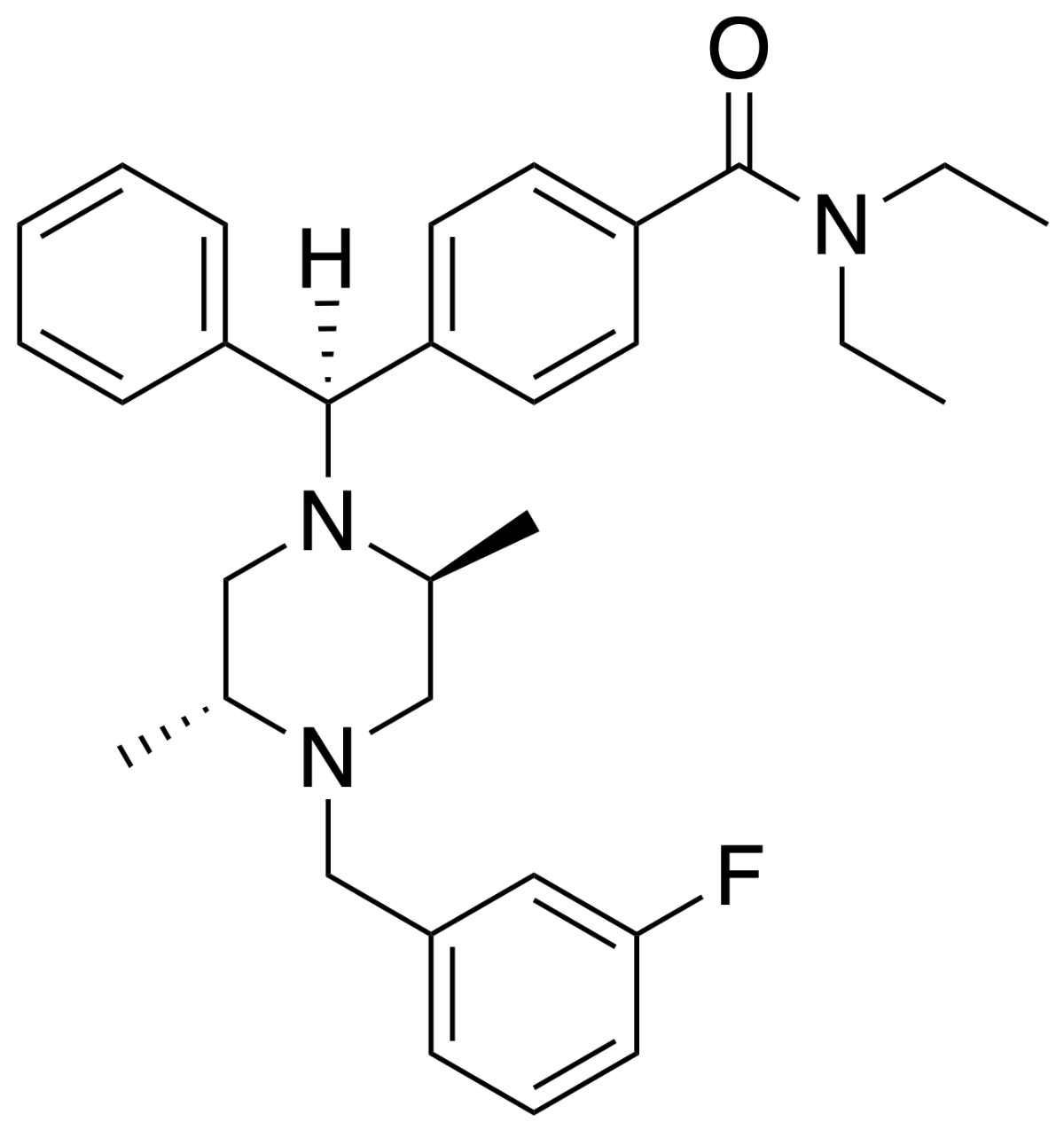
As far as I can see, the SAR of opioids is straight out of Alice through the looking glass, so many different compound exhibit opioid activity.
There is a Ph.D. thesis, by an Indian guy, about DAT inhibitors etc. that is very interesting reading
It is a unique drug, the reason why is it not only metabolizes into morphine, it also metabolizes into 6 monoacetyl morphine, and something else too I think but I can’t remember what, and it crosses the blood brain barrier much quicker than morphine would, causing a stronger rush. Users also report less of a histamine release I believe when using heroin versus morphineIt also makes no sense that heroin is considered a unique compound and not a prodrug for morphine
Hey polar sup dawg!It is a unique drug, the reason why is it not only metabolizes into morphine, it also metabolizes into 6 monoacetyl morphine, and something else too I think but I can’t remember what, and it crosses the blood brain barrier much quicker than morphine would, causing a stronger rush. Users also report less of a histamine release I believe when using heroin versus morphine
It is a unique drug, the reason why is it not only metabolizes into morphine, it also metabolizes into 6 monoacetyl morphine, and something else too I think but I can’t remember what, and it crosses the blood brain barrier much quicker than morphine would, causing a stronger rush. Users also report less of a histamine release I believe when using heroin versus morphine