Image aaa hosted in ImgBB

ibb.co
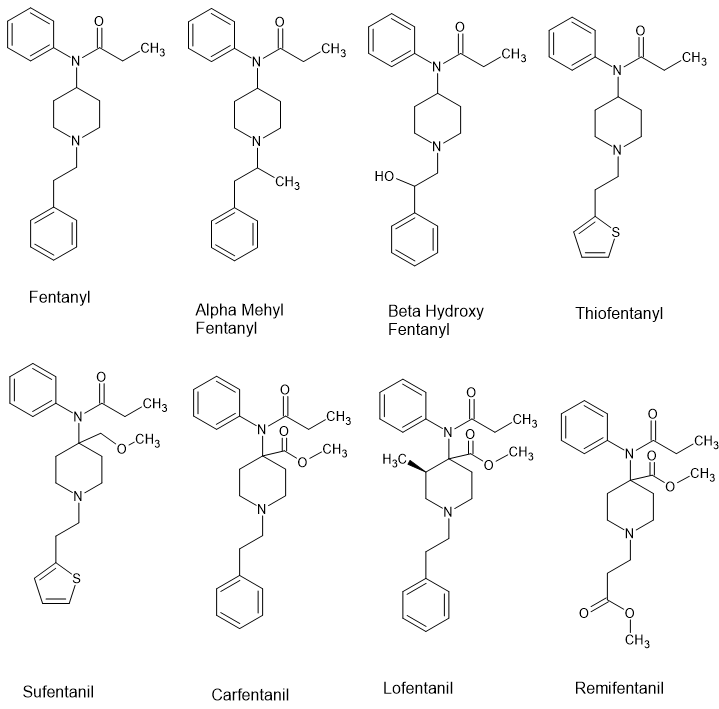
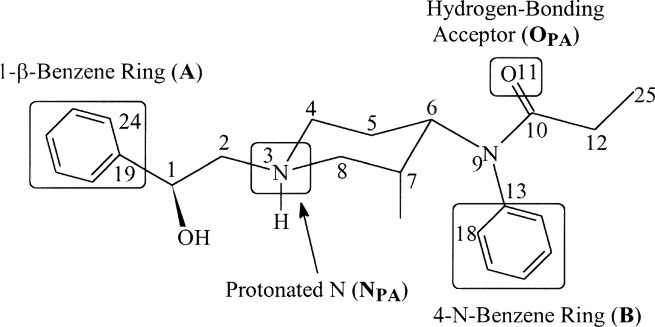
The 5 key moieties (plus 1)
1) α-(ring substituted) 5 or 6 membered aromatic ring (lipophinis, space-filling and ideally with electron density highest in meta positions)
2) hydrogen-bond donator (hydrokyl, ketone, carboxamide and so on)
3) positivelty ionisable function (note does not HAVE to be N, but only rare examples without N discovered thus far).
4) β-(ring-substituted) 6-membered aromatic ring (lipophilic, space-filling and ideally with electron density highest at meta positions)
5) Hydrogen-bond acceptor.
5a) olefinic bond parallel with β-aromatic (see allylprodine, 14-cinnamyloxycodeinone, U-93951, (nor)tilidine)
There is also an addition to Lipinski's 'rule of 5' as it applies to potent mu opioid ligands. The distance between the furthest two atoms
should be 15. If one measures from the para position of one of the benzene rings to the para position of the other, it's 15 atoms in total.
If one then looks at BDPC and counts from the -Br, the length is 15. If one looks at etonitazine, the length from the NO2 to the -CH3 of the
ethoxy is 15. If one counts from the A ring to the terminal -CH3 of the n-pentyl moiety in the most potent etorphine analogue, the length is 15
once again. Even unexpected compounds such as p-Nitro azaprocin are 15 atoms long.