Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
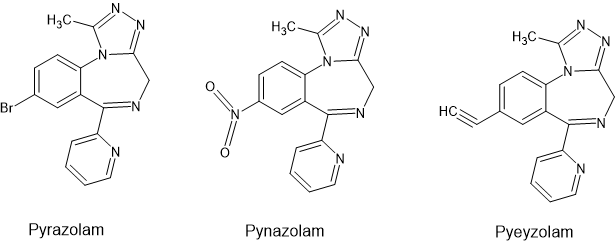
a1 activity produces hypnotic effects. Pynazolam was an experiment in which a scaffold that had almost no a1 affinity was given the 8-substitution most commonly seen in hypnotics (nitro). We discovered that it had potent serotonin releasing activity. We concluded that the reason nitrobenzodiazepines are usually hypnotics is because they have a1 affinity AND increase serotonin levels (both of which cause sleep).
Finally we tried both trimethylsilyl and ethynyl moieties as the 8 position. Both of them emulate 'drunk' very well. The problem is that the effective dose was quite high (25-30mg) and below that they do NOTHING, At first we thought them inactive until we got a bit daring and my wife (of all people) drank 30mg of it in solution and went on to sing all 8 verses of 'Lily the Pink', ordered pizza and fell asleep on the loo.
So while we could have done with trying a few of the pseudohalogens (-CN,-CF3 and so on) we did pretty much find the QSAR when just 1 moiety of the compound was changed.
Oh, and if you remove the 1-methyl, the compounds are almost identical subjectively, but are half the potency.
If I had been given the chance I would have looked into substitutions at the 3 position. Most benzos on the market have a hydroxyl (-OH) or a carboxylic acid (salt) i.e. (-COOH or COOK). The former is generally used to reduce duration, the latter to delay onset. But their are other things one can add. It's those that hold the most promise.



