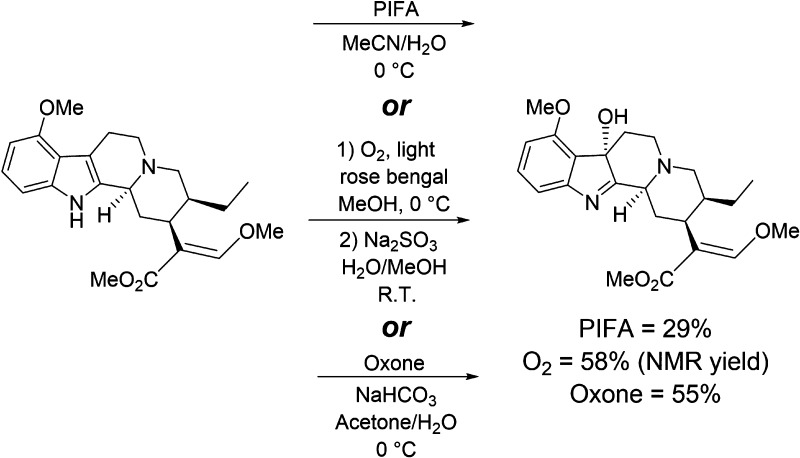
Mitragynine undergoes some degree of auto-oxidation to 7-hydroxymitragynine within the plant, but at very limited rates (the degree of which presumably is based on environmental factors or age). The desired oxidation occurs at C7, increasing potency nearly 20 fold.

Any ideas on how this could be selectively promoted or chemically inspired?

Any ideas on how this could be selectively promoted or chemically inspired?