Montana Industries
Greenlighter
- Joined
- Aug 1, 2015
- Messages
- 13
Hallo my friends,
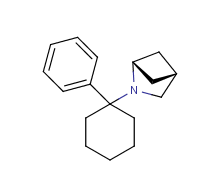
I'm in love with Arylcyclohexylamines and on my search for new Synth routes, I found a way to synth Structurally new Derivatives of PCP and MXE.
MXE also called 3-Meo-2-Oxo-Pce is a very nice Substanz, but has the Problem of different activity because of the two possible stereo isomeres.
The possible derivativa 3-Meo-2,6-diOxo-Pce dont have this problem i think and has maybe new and good psychological activity.
For the PCP derivativa I cant find the correct Name, so I must going to define it.
It have the standard Pcp structure with an ether brige on the Cyclohexane-ring at the 2' 6' position, like the dione.
To the right time i test this compounds, but its nice to hear what do you think about it.
Have thanks and a fucking freaky weekend.
Mfg Mr.Montana
Self drawn pic of the structures:
http://www.bilder-upload.eu/show.php?file=94b73b-1438406441.jpg]
 [/URL
[/URL
I'm in love with Arylcyclohexylamines and on my search for new Synth routes, I found a way to synth Structurally new Derivatives of PCP and MXE.
MXE also called 3-Meo-2-Oxo-Pce is a very nice Substanz, but has the Problem of different activity because of the two possible stereo isomeres.
The possible derivativa 3-Meo-2,6-diOxo-Pce dont have this problem i think and has maybe new and good psychological activity.
For the PCP derivativa I cant find the correct Name, so I must going to define it.
It have the standard Pcp structure with an ether brige on the Cyclohexane-ring at the 2' 6' position, like the dione.
To the right time i test this compounds, but its nice to hear what do you think about it.
Have thanks and a fucking freaky weekend.
Mfg Mr.Montana
Self drawn pic of the structures:
http://www.bilder-upload.eu/show.php?file=94b73b-1438406441.jpg]