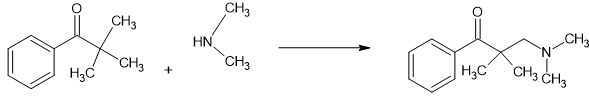Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
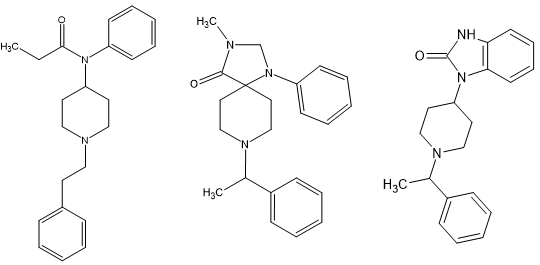
You will notice that ALL of the fentanyl analogues can be readily applied to phenampromide with the papers/patents on propiram are valuable.
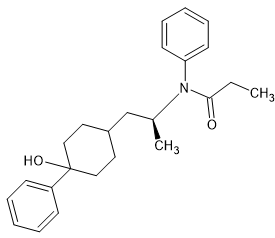
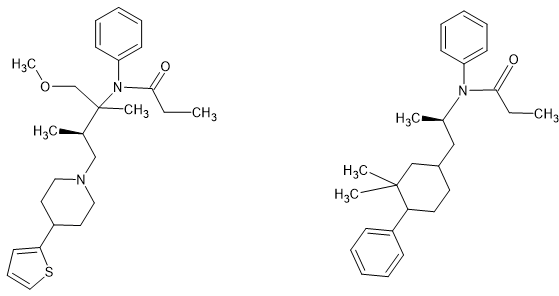
I am the first to admit that it's the (S) enthiomer that is responsible for the opioid activity but it's quite possible to add the methoxymethyl & 2-thienyl moieties of sufentanil but the more I look, the more I realize that the optimal amide is the propenyl. I've looked into dozens of others and all are less potent.
But other than prhenampromide has a chiral centre (that can be removed from the precursor rather rather than the final product which cuts cost a lot.
You may also notice that the 3,3-dimethyl (why would ANYONE make the monomethyl when multiple examples show that the 3,3-dimethyl is almost as active (only the MW lowers it from x2) and also the aromatics follow those of fentanyl.
Now the 1 item I lack is the synthesis of the 3,3-dimethyl-4-phenyl piperidine. I have looked and looked but with no use.
So a little calculation shows that it's possible to reach about x850 M in potency, isn't controlled by by name and isn't watched. In fact it HAS been sold in Europe.
It's all very well to produce the MOST active, but it's cost per dose that matters. That is why I keep telling people to keep on working with Dimethylaminopivalophenone. After all: