SmilexGwG
Bluelighter
- Joined
- Jul 2, 2021
- Messages
- 93
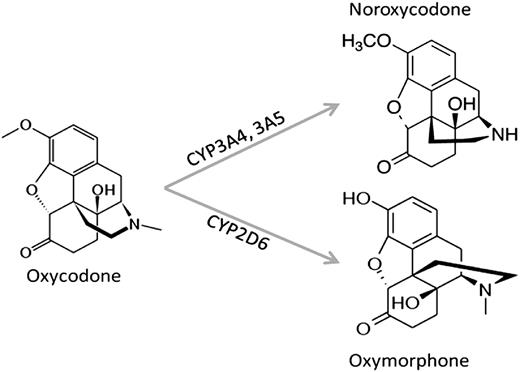
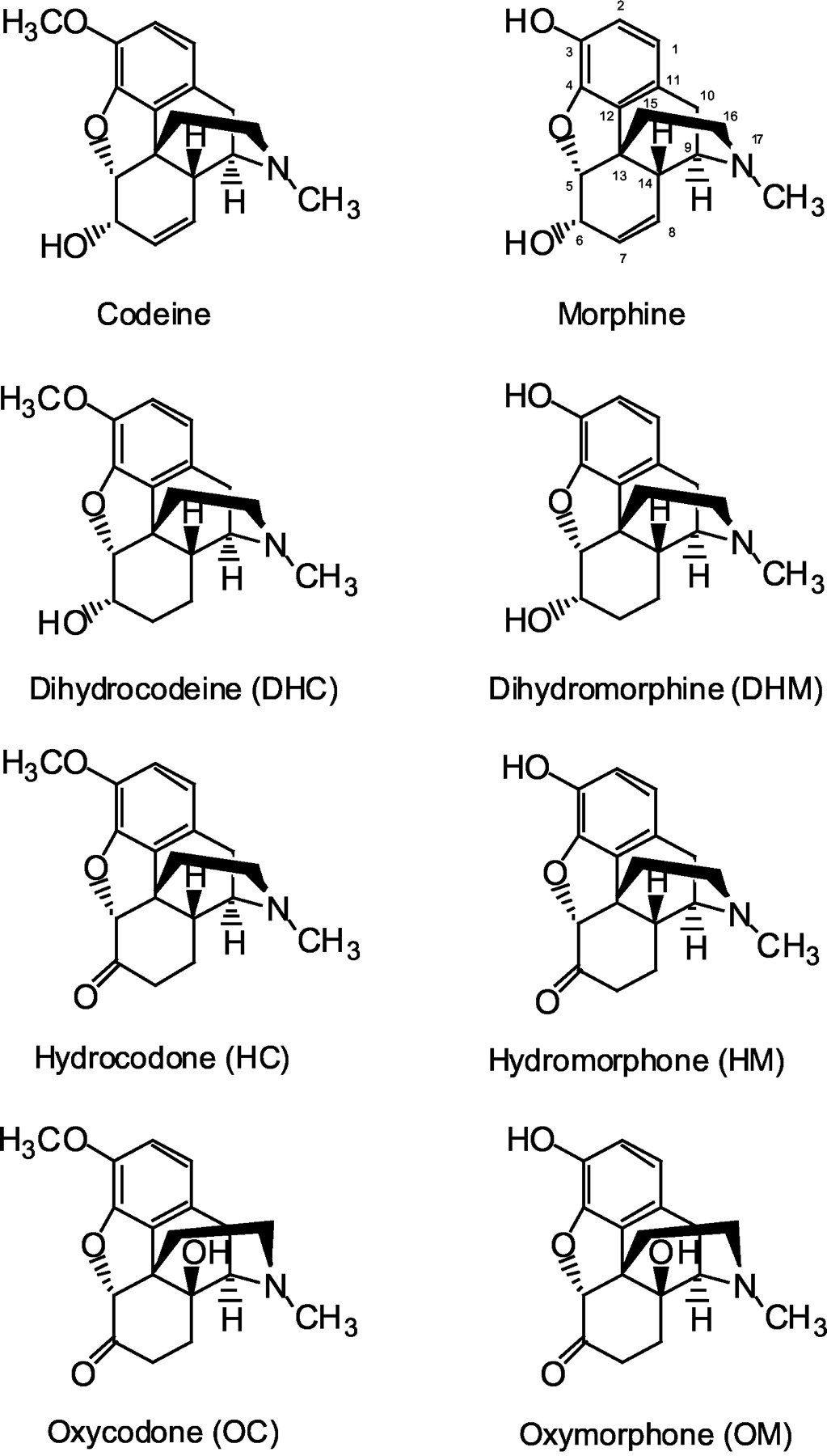
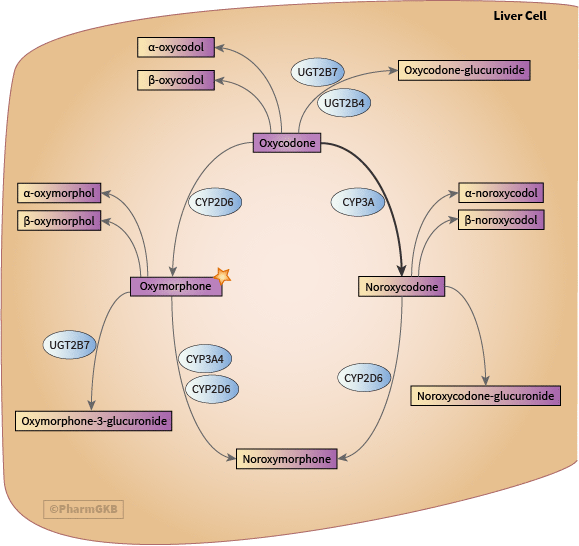
I saw a interesting article a while back on oxycodone being metabolized into oxymorphone and hydrocodone being metabolized into hydromorphone. Obviously not alot of the orginal drug will be fully metabolized but my question is:
Since oxycodones metabolized into oxymorphone via the CYP2D6 enzyme, could a CYP2D6 activator cause more oxymorphone to be formed?
Sorry if this question is stupid or a bit out there, ive been really curious about it lately.
Also im pretty sure this is the right forum but not 100% sorry if this has to be moved
Since oxycodones metabolized into oxymorphone via the CYP2D6 enzyme, could a CYP2D6 activator cause more oxymorphone to be formed?
Sorry if this question is stupid or a bit out there, ive been really curious about it lately.
Also im pretty sure this is the right forum but not 100% sorry if this has to be moved