Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
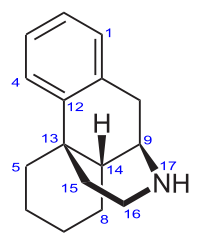
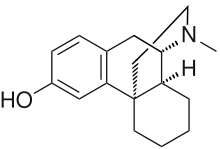
Oreobeiline, closer to morphine than the morphinan skeleton.
Had to edit the title: *closer* to morphine than the morphinan skeleton is. In-fact.
Oreobeiline
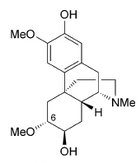
Real interesting. Those bentley compounds have a seven position substitution like the hydroxy on the saturated ring, just not the other on the benzene across. It's also missing that "oxygen cummerbund"
interesting compound, I think of how Bentley used oripavine that isn't found in morphine/codeine poppies, well oreobeiline is found as an alkaloid in Lauraceae, but perhaps a possible precursor in its own right.
Had to edit the title: *closer* to morphine than the morphinan skeleton is. In-fact.
Oreobeiline

Real interesting. Those bentley compounds have a seven position substitution like the hydroxy on the saturated ring, just not the other on the benzene across. It's also missing that "oxygen cummerbund"
interesting compound, I think of how Bentley used oripavine that isn't found in morphine/codeine poppies, well oreobeiline is found as an alkaloid in Lauraceae, but perhaps a possible precursor in its own right.
Last edited: