-
Select Your Topic Then Scroll Down
Alcohol Bupe Benzos Cocaine Heroin Opioids RCs Stimulants Misc Harm Reduction All Topics Gabapentinoids Tired of your habit? Struggling to cope?
Want to regain control or get sober?
Visit our Recovery Support Forums -
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RCs New cannabinoid, ADAMANTYL-THPINACA
- Thread starter roi
- Start date
Sleepwalker18
Bluelighter
- Joined
- Jul 19, 2012
- Messages
- 317
Rolls right off the tongue. And it seems to be a bird attached to the top, might want to discard that. Another Swedish one?
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
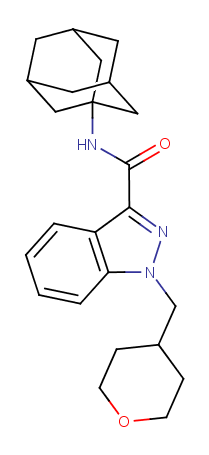
ADAMANTYL-THPINACA (N-adamantyl-1-((tetrahydro-2H-pyran-4-yl)methyl)-1H-indazole-3-carboxamide) is an adamantyl indazole synthetic cannabinoid which shares structural similarities to CUMYL-THPINACA and APINACA. The substance was identified in a seizure of 746 grams of white powder and 2 kilograms of brown/green plant material in Ljubljana by Slovenian police on 28 October 2014. ADAMANTYL-THPINACA was also detected in 49 grams of plant material containing CUMYL-5F-PINACA and in 38 grams of plant material also containing CUMYL-5F-PINACA and ADB-CHMICA. The substance was analytically confirmed by GC-MS, HPLC-TOF and FTIR at the National Forensic Laboratory and by NMR at the Faculty of Chemistry and Chemical Technology, University Ljubljana.
There you go.
cousinskeeter
Bluelighter
- Joined
- Dec 28, 2014
- Messages
- 231
Sounds like the name of a character from Stargate. I like the bird at the top too!
Sleepwalker18
Bluelighter
- Joined
- Jul 19, 2012
- Messages
- 317
I'd wager 1-3mg's for activity, maybe a bit higher. Wish I enjoyed these drugs, might actually have something to say past "That's a nice looking bird on the top of it" 
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
Personally I like potent substances (1-3mg is totally fine for me, with volumetric dosing), however newer cannabinoids such as MDMB-CHMINACA which is active at 50 mcg are definitely way too fucking potent for most people to handle, a barely visible dose is enough to OD!
Due to the Indazole (an Indole will be stronger about 2 times) core, the Amantadine side group (quite bulky, but great for CB1 receptors) and that funky Tetrahydropyrane will make this noid very CB1 selective. I think will be stronger than 5f-AKB48, maybe 3 times...As the pentyl chain is removed for a more nucleophylic group. Fits better in the quaternary structure of CB1. Stronger in CB2 than 5f-AKB48 as well, but will have about 20% of affinity for this receptor.
Possible toxic pyrane as metabolite will be quite rare, as that electronegative oxygen will interact strongly with the lot of SH's in the structure of the receptor.
Maybe 80-90% as potent as AM-2201
Can't wait.
Possible toxic pyrane as metabolite will be quite rare, as that electronegative oxygen will interact strongly with the lot of SH's in the structure of the receptor.
Maybe 80-90% as potent as AM-2201
Can't wait.
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
So many folks are dying to switch to ADAMANTYL-THPINACA - literally.
Those 5Fs and Fluorobenzenes are unacceptably toxic yet they have the desired high CB1 activity.
If ADAMANTYL-THPINACA can provide that high-CB1 activity then it will finally be an alternative to the Fluoronoids.
Those 5Fs and Fluorobenzenes are unacceptably toxic yet they have the desired high CB1 activity.
If ADAMANTYL-THPINACA can provide that high-CB1 activity then it will finally be an alternative to the Fluoronoids.
Duh, adamantane. Never mind.Does that group at the top have a name? It looks neat, but doesn't seem very stable to me.
Known as a diamondoid. Cool.
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
Adamantyl, and yes it is stable: "Adamantane molecules consist of four connected cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free".
The exact opposite of UR-144 and 5F-UR144's extremely strained, ready-to-break, high-energy tetramethylcyclopropane at the same position.
ADAMANTYL-THPINACA's lack of all but CNHO atoms means it ought to pyrolyze clean and metabolize clean, leaving no toxic feeling nor toxic smell on the sweat or breath.
The exact opposite of UR-144 and 5F-UR144's extremely strained, ready-to-break, high-energy tetramethylcyclopropane at the same position.
ADAMANTYL-THPINACA's lack of all but CNHO atoms means it ought to pyrolyze clean and metabolize clean, leaving no toxic feeling nor toxic smell on the sweat or breath.
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
ADAMANTYL-THPINACA was reported by the EMCDDA as part of a seizure in Slovenia in early 2015, but I don't think it was actually sold at all. Would be interesting to see how the tetrahydropyran compares to cyclohexyl (and pentyl, fluoropentyl, fluorobenzyl).
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
Tetrahydropyran, Cyclohexyl and Pentyl will combust cleanly.
Fluoropentyl and Fluorobenzyl will slowly poison the user.
The advantage of ADAMANTYL-THPINACA should be that it does not require a terminal Fluorine to jack the CB1 activation potency.
Terminal Fluorine. Terminal, eventually.
If a non-halogenated group can replace Fluoropentyls and Fluorobenzyls then it will be possible to have a clean high forever, rather than accumulating toxicity.
My friend Jakob is currently testing 5F-AMB but that design is so toxic - particularly to the lungs - that despite its 10x higher potency than 5F-AKB48, the toxic side-effects are about 15x higher.
The lesson here with 5F-AMB is that insanely high potency yielding 10x smaller doses is still not enough to prevent the nasty acute and chronic degenerations caused by this agent.
Certainly not the receptor sides - they correlate with potency - but suprisingly, what seem to be non-receptor-mediated general systemic toxicities also scale with potency in this case.
Those who care will pray that such a potency-toxicity correlation exists among only a few of these cannabinoids.
ADAMANTYL-THPINACA may still have some undesireable side-effects and withdrawal won't ever be fun if it's strong - receptor dynamics.
However, ADAMANTYL-THPINACA contains no halogen atoms - particularly Fluorine.
It can be oxidized to common, safe stuff in the pipe and in the body.
Unlike Fluoronoids which transform into a host of toxic products - many of which due to the tight C-F bond can't ever be broken down by the body - ADAMANTYL-THPINACA is made only of atoms the body can process to completion.
Oxidation of ADAMANTYL-THPINACA's Carbons yields first Carbon Monoxide then Carbon Dioxide.
Oxidation of ADAMANTYL-THPINACA's three Nitrogens yields Nitric Acid then Nitrate salts as the body neutralizes the acid - all of which are highly soluble.
Fluoropentyl and Fluorobenzyl will slowly poison the user.
The advantage of ADAMANTYL-THPINACA should be that it does not require a terminal Fluorine to jack the CB1 activation potency.
Terminal Fluorine. Terminal, eventually.
If a non-halogenated group can replace Fluoropentyls and Fluorobenzyls then it will be possible to have a clean high forever, rather than accumulating toxicity.
My friend Jakob is currently testing 5F-AMB but that design is so toxic - particularly to the lungs - that despite its 10x higher potency than 5F-AKB48, the toxic side-effects are about 15x higher.
The lesson here with 5F-AMB is that insanely high potency yielding 10x smaller doses is still not enough to prevent the nasty acute and chronic degenerations caused by this agent.
Certainly not the receptor sides - they correlate with potency - but suprisingly, what seem to be non-receptor-mediated general systemic toxicities also scale with potency in this case.
Those who care will pray that such a potency-toxicity correlation exists among only a few of these cannabinoids.
ADAMANTYL-THPINACA may still have some undesireable side-effects and withdrawal won't ever be fun if it's strong - receptor dynamics.
However, ADAMANTYL-THPINACA contains no halogen atoms - particularly Fluorine.
It can be oxidized to common, safe stuff in the pipe and in the body.
Unlike Fluoronoids which transform into a host of toxic products - many of which due to the tight C-F bond can't ever be broken down by the body - ADAMANTYL-THPINACA is made only of atoms the body can process to completion.
Oxidation of ADAMANTYL-THPINACA's Carbons yields first Carbon Monoxide then Carbon Dioxide.
Oxidation of ADAMANTYL-THPINACA's three Nitrogens yields Nitric Acid then Nitrate salts as the body neutralizes the acid - all of which are highly soluble.
Last edited:
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
There are a few other non-halogenated 'clean' synthetic cannabinoids besides ADAMANTYL-THPINACA but they tend to have high CB2 activation and relatively low CB1 activation.
The're good for sleep but not particularly interesting or psychedelic.
The Eli-Lilly-developed CHMINACAs are an example of such a family.
The THC-analogues such as HU-210.
And a few more.
Since first starting to learn the synthetic cannabinoid molecular shapes, I remember thinking to myself that it would be good enough to have a bulky group at each end of the Indole/Indazole.
Well, I didn't consider AB-FUBINACA or any of the Cyclohexyls found in some of the CHMINACAs.
Those bulky cycles don't decrease potency but do slow the effect onset a bit.
Frankly, two Adamantyls would likely work well - one on each end.
The're good for sleep but not particularly interesting or psychedelic.
The Eli-Lilly-developed CHMINACAs are an example of such a family.
The THC-analogues such as HU-210.
And a few more.
Since first starting to learn the synthetic cannabinoid molecular shapes, I remember thinking to myself that it would be good enough to have a bulky group at each end of the Indole/Indazole.
Well, I didn't consider AB-FUBINACA or any of the Cyclohexyls found in some of the CHMINACAs.
Those bulky cycles don't decrease potency but do slow the effect onset a bit.
Frankly, two Adamantyls would likely work well - one on each end.
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
A few cannabinoids (such as 5F-UR-144) have been linked to kidney damage, do you know of any research into toxicity of APINACA and its analogues?
Fluorine is present in a million drugs and its existence per se is not exactly a red flag.
Most (all?) synthetic cannabimimetic hospitalizations/deaths seem to be caused by acute overdoses, long term use is not very well understood at all afaik. Especially the cyclohexyls which you consider to be "safe" caused quite a few deaths around the world lately (mostly ADB-CHMINACA and MDMB-CHMICA).
Fluorine is present in a million drugs and its existence per se is not exactly a red flag.
Most (all?) synthetic cannabimimetic hospitalizations/deaths seem to be caused by acute overdoses, long term use is not very well understood at all afaik. Especially the cyclohexyls which you consider to be "safe" caused quite a few deaths around the world lately (mostly ADB-CHMINACA and MDMB-CHMICA).
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
1. A few containing the tetramethylcyclopropane group. The percent of individuals damaged is very tiny, leading to the obvious conclusion that kidney vulnerability is genetic/environmental.
2. I've heard similar phrases on many forums. Yet making a statement like that without a use context is damaging. The use context of Fluoronoids is smoking/thermal vaporization. Even with care in a vaporizer pipe, 5F-AMB produces both the fluoro-stink on sweat [toxic metabolism/excretion] and physical symptoms of toxicity. Notably, my friend Jakob just tested 5F-AMB on tobacco in a smoking pipe with direct flame and gently vaporized in a vaporization pipe. In both cases the toxic stink was notable exuding from sweat almost 24 hours later, but the day after he vaporized instead of combusting it, the 5F-AMB produced less overall stink on sweat and breath, and the smell itself was missing the worst 'toxic stench' that he was used to from once-an-evening 5F-AKB48.
The observation that both of these powders has no inherent smell means that the toxic scent on sweat and breath is due to pyrolysis/metabolism.
Correctly, most medical troubles caused by these synthetic cannabinoids are CB-receptor related. They can be lethal and they can be dramatic.
However, despite the receptor drama receptors re-regulate.
The ugly problems creep up slower as a user's body becomes ever more filled with fluorinated toxins, which due to the strong C-F bond can't be broken down completely, nor completely excreted.
The rule is that if the liver can't easily break it down and the kidneys can't easily excrete it, that the body tends to dispose of a poison by sweating it out of the skin and lungs (breath).
The fact that a few milligrams of 5F-AMB's partial-breakdown products can still be smelled on the breath & sweat twenty hours later means that the drug is so hard to break down that the liver/kidneys have accomplished very little against those few milligrams in so many hours.
The promise of ADAMANTYL-THPINACA is that it will break down easily and quickly relative to the fluoropoisons.
You are indeed correct that the high-CB2 CHMINACAs can cause major problems, as can the high-CB1 activators such as JWH-018, AM-2201, 5F-AMB.
The problems caused are receptor related however - agonism/antagonism/withdrawal.
2. I've heard similar phrases on many forums. Yet making a statement like that without a use context is damaging. The use context of Fluoronoids is smoking/thermal vaporization. Even with care in a vaporizer pipe, 5F-AMB produces both the fluoro-stink on sweat [toxic metabolism/excretion] and physical symptoms of toxicity. Notably, my friend Jakob just tested 5F-AMB on tobacco in a smoking pipe with direct flame and gently vaporized in a vaporization pipe. In both cases the toxic stink was notable exuding from sweat almost 24 hours later, but the day after he vaporized instead of combusting it, the 5F-AMB produced less overall stink on sweat and breath, and the smell itself was missing the worst 'toxic stench' that he was used to from once-an-evening 5F-AKB48.
The observation that both of these powders has no inherent smell means that the toxic scent on sweat and breath is due to pyrolysis/metabolism.
Correctly, most medical troubles caused by these synthetic cannabinoids are CB-receptor related. They can be lethal and they can be dramatic.
However, despite the receptor drama receptors re-regulate.
The ugly problems creep up slower as a user's body becomes ever more filled with fluorinated toxins, which due to the strong C-F bond can't be broken down completely, nor completely excreted.
The rule is that if the liver can't easily break it down and the kidneys can't easily excrete it, that the body tends to dispose of a poison by sweating it out of the skin and lungs (breath).
The fact that a few milligrams of 5F-AMB's partial-breakdown products can still be smelled on the breath & sweat twenty hours later means that the drug is so hard to break down that the liver/kidneys have accomplished very little against those few milligrams in so many hours.
The promise of ADAMANTYL-THPINACA is that it will break down easily and quickly relative to the fluoropoisons.
You are indeed correct that the high-CB2 CHMINACAs can cause major problems, as can the high-CB1 activators such as JWH-018, AM-2201, 5F-AMB.
The problems caused are receptor related however - agonism/antagonism/withdrawal.
Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
ADAMANTYL-THPINACA: Analysis I
Before I drill down into the details of ADAMANTYL-THRPINACA and how it compares to other designs, it's valuable to observe a graphic image showing the classic synthetic Cannabinoid structural features:
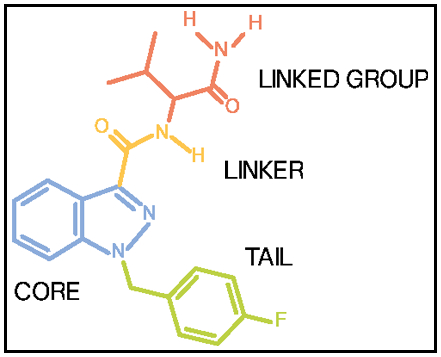
Synthetic Cannabinoid Group Terminology
One more piece of terminology is required to get a handle on these molecules' structure-activity relationships: Electrostatic Charge Distribution:
Wikipedia: Charge Density
Tutorial: Electrostatic Potential Maps
Cannabinoids work not by the typical mechanism of Hydrogen Bonding but rather, activate CB-receptors by a very different mechanism: Stacking:
3-Indolyl-1-naphthylmethanes: New Cannabimimetic Indoles Provide Evidence for Aromatic Stacking Interactions with the CB1 Cannabinoid Receptor
Identification of Essential Cannabinoid-binding Domains
The Difference between the CB1 and CB2 Cannabinoid Receptors at Position 5.46 Is Crucial for the Selectivity of WIN55212-2 for CB2
The Importance of Hydrogen Bonding and Aromatic Stacking to the Affinity and Efficacy of Cannabinoid Receptor CB2 Antagonist
Thus Cannabinoid molecules are free of Hydrophilic Carboxyls, etc. and are almost exclusively fat-soluble.
Their Tails and Linked Groups are always Lipophilic & Hydrophobic.
ADAMANTYL-THPINACA has a small point of rather high terminal electronegativity due to the Oxygen atom at the distal end of its Cyclohexyl:
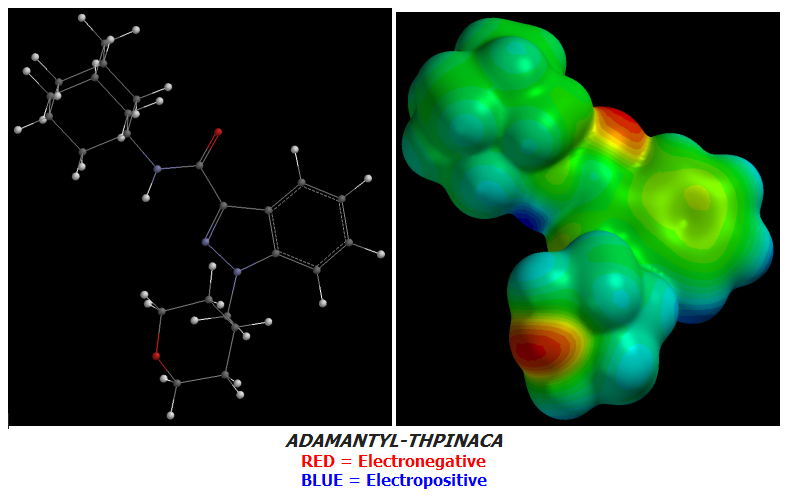
In the last few days I have designed a few superior & more terminally-Electronegative Cannabinoid candidates based on the structure of APINACA which ADAMANTYL-THPINACA itself is only one Tail-difference away from. My designs also modify only the Tail of APINACA.
The Adamantyl-Cannabinoid family:
Adamantyl Cannabinoids rely upon Tail terminal Electronegativity for both their absolute potency and CB1:CB2 activation ratio.
Our Future shall not be our Past but only by understanding History may we avoid its mistakes and craft a better Future for ourselves and any Descendents. This post is about the world's public Adamantyl Story beginning with APICA and ending yesterday with ADAMANTYL-THPINACA. However, rather than ending it turns out the Adamantyl-Cannabinoid train track was still under construction, having not yet reached its ultimate destinations.
Today a new section of that track is to be hammered into place by the blood, sweat and tears of one illegitimate designer.
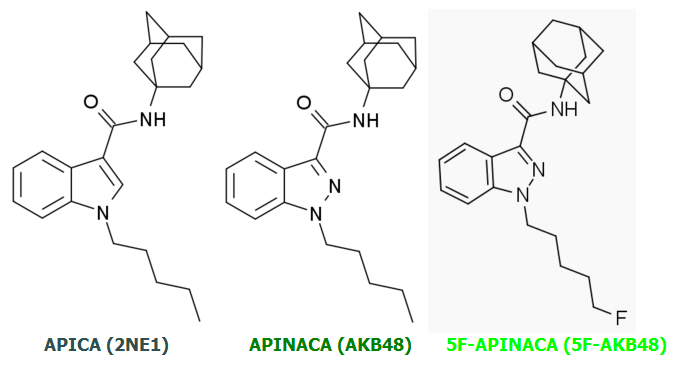
APINACA achieves a slight increase in potency and CB1:CB2 activation ratio relative to APICA but these increases are small and incremental.
5F-APINACA is a quantum leap ahead of its two predecessors in potency and CB1:CB2 ratio due to a single revolutionary change.
This same change transformed the already-potent JWH-018 into monstrous AM-2201: Tail Terminal Fluorination.
Specifically: A massive increase in Tail Terminal Electronegativity relative to their non-Fluorinated parent molecules.
Below are correspondent Electrostatic Potential Maps of the three molecules above.
These Cannabinoids are freshly-baked from their respective Energy-Minimized forms by Quantum Electromechanical Simulation & Rendering.
It took ~32 minutes to complete each molecular (QED Simulation + Rendering) on my Core2Duo E6300 using avg. ~96% of both cores during QED Simulation & ~100% of one core during Rendering.
QED Simulation: ~75% total clock-time.
Rendering: ~25% total clock-time.
.
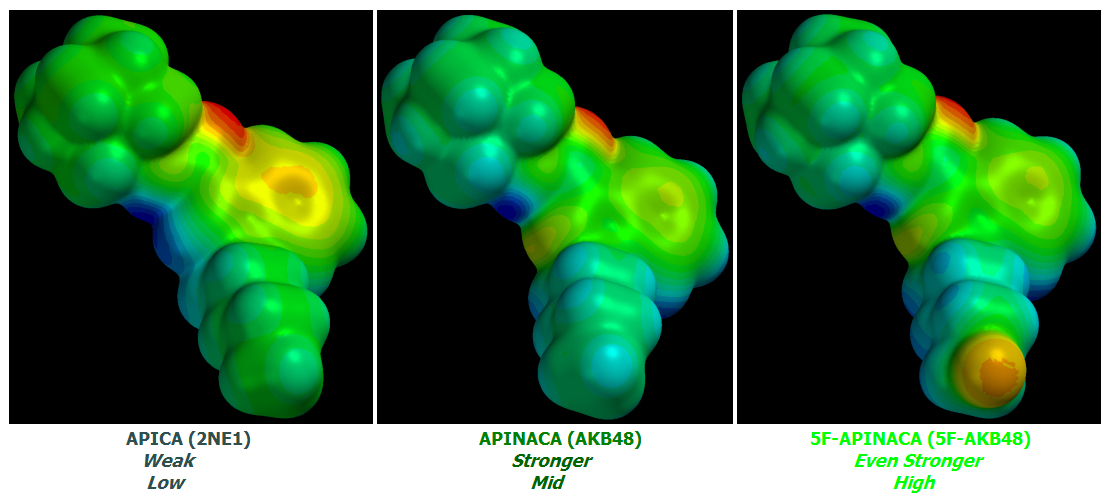
The small potency increase from APICA to APINACA is due to a non-terminal effect: the removal of a small quantity of charge from its Core to its Linked Group due to Core Indole to Indazole conversion. APINACA's loss of one Hydrogen at that atomic position due to the substituted Nitrogen's Trivalency relative to the replaced Carbon's Tetravalency increases the effect.
APINACA's Tail is less Electronegative than APICA's but not by enough to ruin the Core substitutional effects.
Nonetheless, these differences are tiny quibbles compared to 5F-APINACA's Quantum leap in potency and CB1:CB2 activation ratio.
Now for the next comparison: 5F-APINACA (5F-AKB48) versus ADAMANTYL-THPINACA:
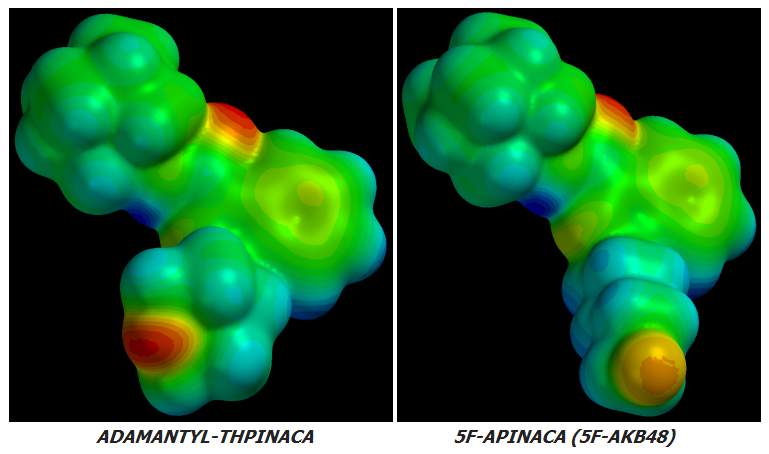
Note how the Energy-Minimized form of ADAMANTYL-THPINACA adopts a unique bend in its Ethyl Linker between the Cyclohexyl Tail and Indazole Core.
This unique bend and cylic Tail are two radical departures from the Adamantyl family's otherwise linear Alkyl Tail feature except one.
The only other Adamantyl Cannabinoid exhibiting this bend - and the cyclic tail which also accompanies it - is FUB-APINACA (FUB-AKB48):
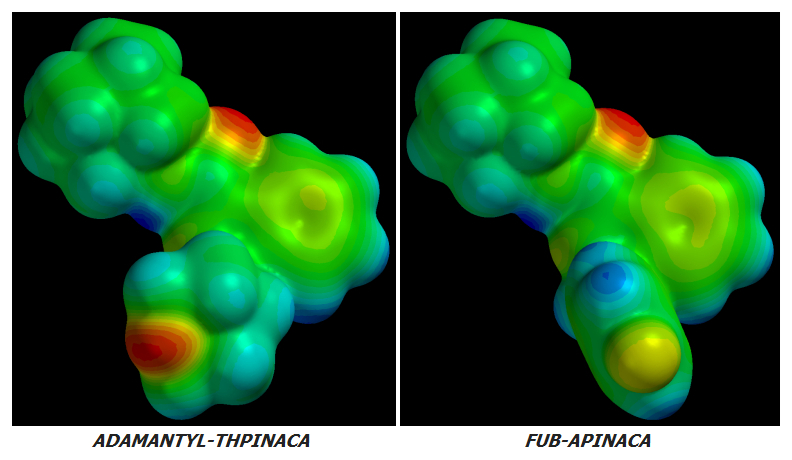
Note how ADAMANTYL-THPINACA's Energy-Minimized Cyclohexyl group bends in the opposite direction to FUB-APINACA's Benzyl group.
1.1 The Ethyl Linker is bend-flexible: it allows the attached cycle whether Benzyl or Cyclohexyl to bend relative to the rest of the Cannabinoid molecule. That bend angle can change as this type of Cannabinoid fits into CB-receptors due to the Ethyl Linker's flexibility.
1.2 The Ethyl Linker is rotatable. Note that ADAMANTYL-THPINACA's Cyclohexyl is rotated clockwise ~20° relative to FUB-APINACA's Benzyl. That rotation was not inherent when I assembled these two molecules. It spontaneously occurred after I instructed the QED software to minimize these molecules' energy. This means the Ethyl Linker is easily rotatable under the small Electrostatic pressures present in the rest of the molecule. CB-receptors exert many more and quantitatively-larger Electrostatic pressures as these molecules fit into them.
2. ADAMANTYL-THPINACA's terminal Oxygen shows bright [RED] indicating very high Electronegativity while FUB-APINACA's terminal Fluorine is merely [LEMON-YELLOW] which indicates much lower Electronegativity.
3. One might be led to conclude that ADAMANTYL-THPINACA will have higher potency and CB1:CB2 Receptor Activation Ratio than FUB-APINACA; however, ADAMANTYL-THPINACA's [RED] Oxygen is buried in its Cyclohexyl ring while FUB-APINACA's [LEMON-YELLOW] Fluorine sticks out.
4. Despite FUB-APINACA's terminal Fluorine atom having much lower Electronegativity than ADAMANTYL-THPINACA's termocyclic Oxygen, FUB-APINACA's Fluorine sticks out aggressively like a Molecular Penis thus exposing itself to far more interaction with Female [Yin] CB-Receptors. Receptors are hollow areas corresponding to Female [Yin] organs while their ligands correspond to Male [Yang] parts.
5. My subjective call is that due to being buried as part of a Cyclohexyl ring - and despite its higher Electronegativity - ADAMANTYL-THPINACA's net terminal Electronegative effect at CB-Receptors will be lower than that of FUB-APINACA's. Thus its potency and CB1:CB2 Receptor Activation Ratio will also be lower.
6. ADAMANTYL-THPINACA's designer(s) earn my respect for creating a CB-agonist with decent terminal Electronegativity while avoiding the use of a toxic Halogen such as Fluorine or Chlorine. Such a design takes real creativity and/or lots of in-Silico guess/test cycles as I myself have recently found out. Instead they used a Pyrosafe Oxygen atom which will not result in toxic product formation when the molecule is heated to or past its thermal decomposition temperature. This is ADAMANTYL-THPINACA's most valuable Harm-Reduction feature.
7. Finally, ADAMANTYL-THPINACA's Tail is a Cyclohexyl ring rather than FUB-APINACA's Benzyl. This is a large difference thus making effect extrapolation difficult but not necessarily irrelevant or impossible.
We do have limited forum reports of FUB-APINACA human test results from which to extrapolate the potential effects of ADAMANTYL-THPINACA:
Yet FUB-APINACA is still the most similar Adamantyl-Cannabinoid to ADAMANTYL-THPINACA thus the comparison.
Further QED simulations, renderings and analyses for new and novel Cannabinoid molecular designs which far exceed those above in terminal Electronegativity will be presented by the end of this Sunday in posts on the following thread: Rational Cannabinoid Design: From Intoxicant to Chemical Weapon.
Before I drill down into the details of ADAMANTYL-THRPINACA and how it compares to other designs, it's valuable to observe a graphic image showing the classic synthetic Cannabinoid structural features:

Synthetic Cannabinoid Group Terminology
One more piece of terminology is required to get a handle on these molecules' structure-activity relationships: Electrostatic Charge Distribution:
Wikipedia: Charge Density
Tutorial: Electrostatic Potential Maps
3-Indolyl-1-naphthylmethanes: New Cannabimimetic Indoles Provide Evidence for Aromatic Stacking Interactions with the CB1 Cannabinoid Receptor
Identification of Essential Cannabinoid-binding Domains
The Difference between the CB1 and CB2 Cannabinoid Receptors at Position 5.46 Is Crucial for the Selectivity of WIN55212-2 for CB2
The Importance of Hydrogen Bonding and Aromatic Stacking to the Affinity and Efficacy of Cannabinoid Receptor CB2 Antagonist
Thus Cannabinoid molecules are free of Hydrophilic Carboxyls, etc. and are almost exclusively fat-soluble.
Their Tails and Linked Groups are always Lipophilic & Hydrophobic.
ADAMANTYL-THPINACA has a small point of rather high terminal electronegativity due to the Oxygen atom at the distal end of its Cyclohexyl:

In the last few days I have designed a few superior & more terminally-Electronegative Cannabinoid candidates based on the structure of APINACA which ADAMANTYL-THPINACA itself is only one Tail-difference away from. My designs also modify only the Tail of APINACA.
The Adamantyl-Cannabinoid family:
5Cl-AKB48
5F-AKB48 [1400742-13-3]
AB-001 [1345973-49-0]
ADAMANTYL-THPINACA
AKB48 (APINACA) [1345973-53-6]
APICA (2NE1) [1345973-50-3]
FUB-AKB48 (FUB-APINACA)
STS-135 [1354631-26-7]
5F-AKB48 [1400742-13-3]
AB-001 [1345973-49-0]
ADAMANTYL-THPINACA
AKB48 (APINACA) [1345973-53-6]
APICA (2NE1) [1345973-50-3]
FUB-AKB48 (FUB-APINACA)
STS-135 [1354631-26-7]
Adamantyl Cannabinoids rely upon Tail terminal Electronegativity for both their absolute potency and CB1:CB2 activation ratio.
Our Future shall not be our Past but only by understanding History may we avoid its mistakes and craft a better Future for ourselves and any Descendents. This post is about the world's public Adamantyl Story beginning with APICA and ending yesterday with ADAMANTYL-THPINACA. However, rather than ending it turns out the Adamantyl-Cannabinoid train track was still under construction, having not yet reached its ultimate destinations.
Today a new section of that track is to be hammered into place by the blood, sweat and tears of one illegitimate designer.

APINACA achieves a slight increase in potency and CB1:CB2 activation ratio relative to APICA but these increases are small and incremental.
5F-APINACA is a quantum leap ahead of its two predecessors in potency and CB1:CB2 ratio due to a single revolutionary change.
This same change transformed the already-potent JWH-018 into monstrous AM-2201: Tail Terminal Fluorination.
Specifically: A massive increase in Tail Terminal Electronegativity relative to their non-Fluorinated parent molecules.
Below are correspondent Electrostatic Potential Maps of the three molecules above.
These Cannabinoids are freshly-baked from their respective Energy-Minimized forms by Quantum Electromechanical Simulation & Rendering.
It took ~32 minutes to complete each molecular (QED Simulation + Rendering) on my Core2Duo E6300 using avg. ~96% of both cores during QED Simulation & ~100% of one core during Rendering.
QED Simulation: ~75% total clock-time.
Rendering: ~25% total clock-time.

APINACA's Tail is less Electronegative than APICA's but not by enough to ruin the Core substitutional effects.
Nonetheless, these differences are tiny quibbles compared to 5F-APINACA's Quantum leap in potency and CB1:CB2 activation ratio.
Now for the next comparison: 5F-APINACA (5F-AKB48) versus ADAMANTYL-THPINACA:

Note how the Energy-Minimized form of ADAMANTYL-THPINACA adopts a unique bend in its Ethyl Linker between the Cyclohexyl Tail and Indazole Core.
This unique bend and cylic Tail are two radical departures from the Adamantyl family's otherwise linear Alkyl Tail feature except one.
The only other Adamantyl Cannabinoid exhibiting this bend - and the cyclic tail which also accompanies it - is FUB-APINACA (FUB-AKB48):

Note how ADAMANTYL-THPINACA's Energy-Minimized Cyclohexyl group bends in the opposite direction to FUB-APINACA's Benzyl group.
1. The CB-Receptor effects of the Ethyl Linker's different bend-angle are too complex for me to predict with any chance of success so I will stick to the Electronegativity-based predictions below. Before leaving the Ethyl Linker behind however, it is valuable to note two unarguable characteristics it possesses:
1.1 The Ethyl Linker is bend-flexible: it allows the attached cycle whether Benzyl or Cyclohexyl to bend relative to the rest of the Cannabinoid molecule. That bend angle can change as this type of Cannabinoid fits into CB-receptors due to the Ethyl Linker's flexibility.
1.2 The Ethyl Linker is rotatable. Note that ADAMANTYL-THPINACA's Cyclohexyl is rotated clockwise ~20° relative to FUB-APINACA's Benzyl. That rotation was not inherent when I assembled these two molecules. It spontaneously occurred after I instructed the QED software to minimize these molecules' energy. This means the Ethyl Linker is easily rotatable under the small Electrostatic pressures present in the rest of the molecule. CB-receptors exert many more and quantitatively-larger Electrostatic pressures as these molecules fit into them.
2. ADAMANTYL-THPINACA's terminal Oxygen shows bright [RED] indicating very high Electronegativity while FUB-APINACA's terminal Fluorine is merely [LEMON-YELLOW] which indicates much lower Electronegativity.
3. One might be led to conclude that ADAMANTYL-THPINACA will have higher potency and CB1:CB2 Receptor Activation Ratio than FUB-APINACA; however, ADAMANTYL-THPINACA's [RED] Oxygen is buried in its Cyclohexyl ring while FUB-APINACA's [LEMON-YELLOW] Fluorine sticks out.
4. Despite FUB-APINACA's terminal Fluorine atom having much lower Electronegativity than ADAMANTYL-THPINACA's termocyclic Oxygen, FUB-APINACA's Fluorine sticks out aggressively like a Molecular Penis thus exposing itself to far more interaction with Female [Yin] CB-Receptors. Receptors are hollow areas corresponding to Female [Yin] organs while their ligands correspond to Male [Yang] parts.
5. My subjective call is that due to being buried as part of a Cyclohexyl ring - and despite its higher Electronegativity - ADAMANTYL-THPINACA's net terminal Electronegative effect at CB-Receptors will be lower than that of FUB-APINACA's. Thus its potency and CB1:CB2 Receptor Activation Ratio will also be lower.
6. ADAMANTYL-THPINACA's designer(s) earn my respect for creating a CB-agonist with decent terminal Electronegativity while avoiding the use of a toxic Halogen such as Fluorine or Chlorine. Such a design takes real creativity and/or lots of in-Silico guess/test cycles as I myself have recently found out. Instead they used a Pyrosafe Oxygen atom which will not result in toxic product formation when the molecule is heated to or past its thermal decomposition temperature. This is ADAMANTYL-THPINACA's most valuable Harm-Reduction feature.
7. Finally, ADAMANTYL-THPINACA's Tail is a Cyclohexyl ring rather than FUB-APINACA's Benzyl. This is a large difference thus making effect extrapolation difficult but not necessarily irrelevant or impossible.
We do have limited forum reports of FUB-APINACA human test results from which to extrapolate the potential effects of ADAMANTYL-THPINACA:
ChemsRUS: AFB-48/fub-akb48
ChemsRUS: FUB-AKB-48
Flashback Forums: FUB-AKB48
Forum reports indicate that FUB-APINACA has good potency but are as yet too few & vague to draw a consistent picture of its effects.ChemsRUS: FUB-AKB-48
Flashback Forums: FUB-AKB48
Last edited: