Thomas Varley, Neuroscientist
Do psychedelics trigger neurogenesis? Here's what we know.
Neurogenesis (the process by which the brain grows new neurons) has become something of a scientific buzzword recently, both in and out of psychedelic circles. It’s not hard to find supplements claiming that, through some pharmaceutical wizardry, you can harness the “power of neurogenesis.” Many psychedelic blogs have gotten very excited by the prospect that drugs like psilocybin might cause neurogenesis, hoping to generate momentum for the psychedelics-as-real-medicines cause.
After all, who could be against neurogenesis? Growing new brain cells does sound like the first step on the path to super powers, or at the very least, seriously enhancing abilities that we already have. But what is neurogenesis? Do psychedelics really cause it? If they do, what doors might that open up?
The vast, vast majority of neurogenesis happens before we are born: you come into this world with most of the neurons you’ll ever have, and over the course of your life they slowly die off. It has long been thought that the number of neurons you’d ever have is fixed at birth, but now we know that’s not quite true: adult neurogensis has been found to happen in a few select brain regions.
Even though adult neurogenesis is happening in the brain, it’s not happening a whole lot, and only in some pretty particular areas. The cerebral cortex (where most of the highest-level stuff happens) isn’t even on the map here. Sadly, neurogenesis isn’t going to turn you into some kind of mega-brain: most of your nervous system will remain unaffected by drugs that trigger neurogenesis. As far as we know, if you have damage to part of your cerebral cortex, taking psychedelics is unlikely to regrow the affected areas.
So should we all pack up and go home?
Not quite―just because neurogenesis isn’t as wide-spread or powerful as popular coverage might make it seem, there’s still reason to get excited. Of the few locations for neurogenesis, what’s happening in the dentate gyrus of the hippocampus is probably the most interesting, at least to those of us interested in psychedelic neuroscience. If those words sound like some kind of Harry Potter spell to you, you’re not alone and you may find some comfort knowing that generations of students have wept their way through neuroanatomy classes working on these same terms. The hippocampus is involved with many different aspects of cognition, but, at the risk of oversimplifying things, its primary role seems to be regulating both learning and memory.
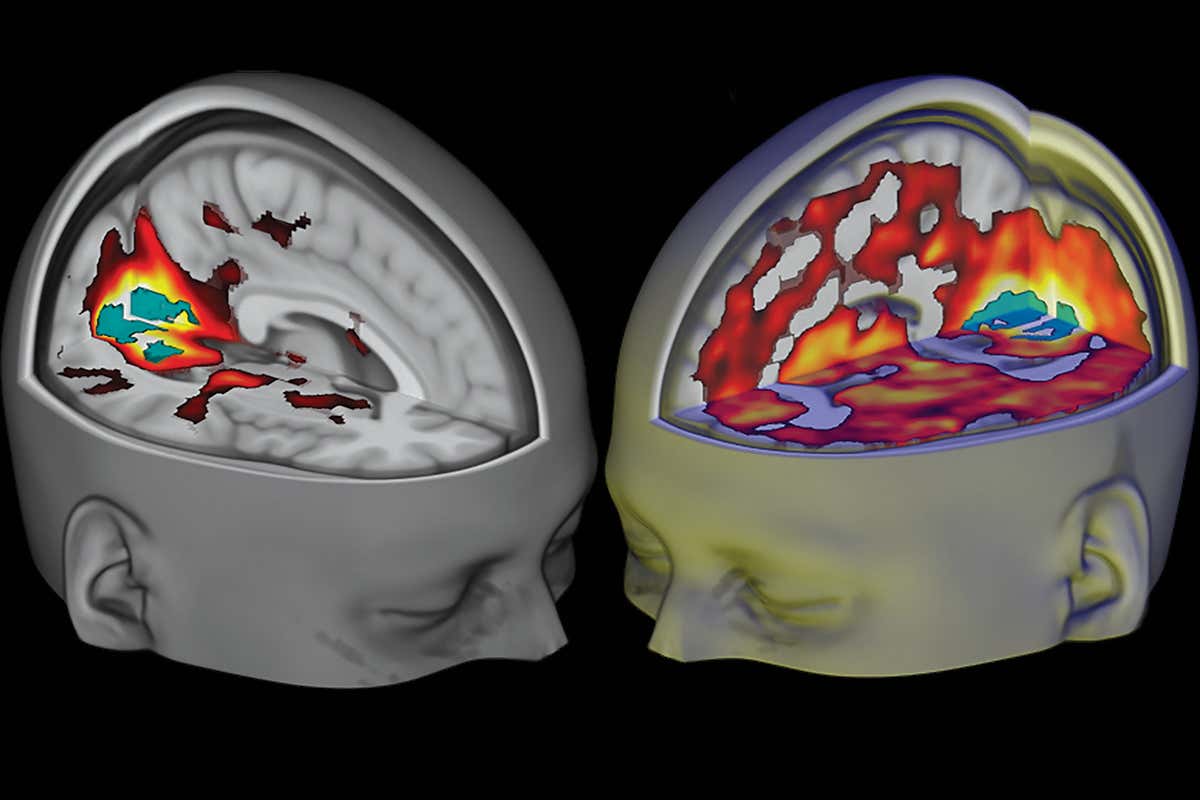
Damage to the hippocampus can result in a variety of interesting and unpleasant effects, including permanent anterograde amnesia (the inability to form new memories), and it’s one of the first places damage from Alzheimer’s Disease manifests in the brain. For reasons that remain unclear, severe cases of major depression are associated with atrophy of the hippocampus, sometimes by as much as 20 percent, which may explain why, as anyone who has suffered from severe depression knows, being depressed is more than just being down all the time. It comes with its own unique constellation of cognitive effects, including memory problems and issues with focusing and concentration. This last finding is particularly interesting when we add in the fact that the balance of evidence suggests that exposure to psychedelics can, in fact, enhance neurogenesis in this region. This has some pretty profound implications for neuroscience and medicine.
It’s actually a little-known fact that there’s been some research that suggests psychedelics can enhance the natural ability to learn new behaviors and form associations. So far, all the work has been done with animals (rabbits and rats, mostly), but the promise is there.
A CORNAL CUTTING OF A MACAQUE BRAIN, USING A NISSL STAIN. THE HIPPOCAMPUS IS CIRCLED
AND THE DENTATE GYRUS IS LABELED
Two studies using LSD found that the psychedelic enhanced the rate at which rabbits learned a new conditioned behavior, and that higher doses resulted in faster learning. The same researchers found that MDMA, MDA, and DOM all did as well. A more recent study using psilocybin found similar results, albeit only at low doses. It’s hard to draw any strong conclusions from a handful of studies like this―it’s a long way from simple associative learning in a rabbit or rat, to a complex human behavior (like playing the piano), but it’s a start. For researchers interested in treating debilitating psychological conditions like depression using psychedelic medicines, these are enormously promising results.
Why this doesn’t get talked about more in psychedelic circles is beyond me.
So what does this have to do with the idea of neurogenesis? Neurogensis is thought to be one of the mechanisms by which this kind of learning might occur. There have been studies that suggest that, for at least some kind of learning, neurogenesis in the hippocampus may be a key part of the acquisition of new behaviors and pattern recognition. In the interest of fairness, it is worth noting that not every study has validated this theory―there’s still quite a bit of science to be done, but the groundwork has been laid. The same team that was researching the effects of psilocybin on learning in rats found signs of new neural growths in the hippocampus in the rats that had been given the low dose psychedelic treatment and learned the new behavior faster. Unfortunately, as of now, this is the only study that has found a psychedelic triggered neurogenesis AND enhanced learning behavior.
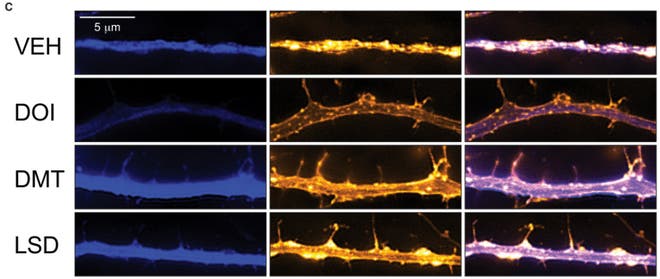
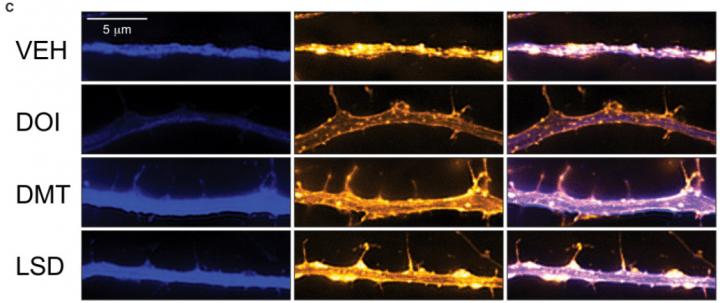
Don’t despair though, there is some circumstantial evidence that should be of interest to those banking on this theory of psychedelic neurogenesis. It has been known for quite a while that the receptor that psychedelic drugs target (the Serotonin 2A receptor) helps regulate the production of a molecule called Brain-Derived Neurotrophic Factor (BDNF, for short). Activate the receptor, and the brain secretes more BDNF. My own (unpublished) research found that that the psychedelic drug DPT (a close analogue of the more famous DMT) increased signs of BDNF in the brains of adult zebrafish, and studies using neurons in a dish and the drug DOI found similar results.
BDNF helps regulate neurogenesis and neuroplasticity: mice that have been artificially rendered unable to produce BDNF show severely distorted nervous systems, with behaviors thought to be related to psychiatric illnesses like eating disorders and OCD. Using genetic techniques to increase BDNF expression can enhance neurogenesis in certain brain regions as well. So far, no one has shown that BDNF causes increased learning capability directly, although participating in learning tasks causes a rapid increase in hippocampal BDNF expression in rats.

My (tentative) hypothesis is that psychedelic drugs can enhance learning and memory capabilities by, at least partially, increasing the amount of BDNF (and related growth-factors) in the brain through activation of the Serotonin 2A receptor. The evidence for this idea is circumstantial right now―so far there hasn’t been a study that combines all of these different moving parts. At the very least, you would need to show that exposure to something like LSD increased how quickly an animal learned a new task, that levels of BDNF in the brain went up, and there was evidence of increased neurogenesis in the hippocampus. This is a fairly tall order, although not one that’s impossible, not by a long shot. All of the individual parts are well within the capabilities of modern science; it’s getting someone to throw time and money behind the question that’s the trick.
Interestingly, in 2016, the Beckley Foundation, working with scientists at the Sant Pau Institute for Biomedical Research in Spain announced findings that two of the key components in Ayahuasca, harmine and tetrahydroharmine stimulate the differentiation of stem cells into healthy neurons when they’re cultured in a dish. There’s still a lot of work to be done on this topic: researchers are moving ahead studying whether the same effect will be seen in living animals, and, if the findings are replicated, these findings would have big implications for the science of neurogenesis.
How these two molecules might stimulate neurogenesis is an open question: unlike drugs like psilocybin and LSD, which act directly at the 5-HT2A receptor, harmine and tetrahydroharmine act as inhibitors of the enzyme monoamine oxidase (MAO), which degrades natural neurotransmitters like serotonin and dopamine. It may be that, when MAO is inhibited, the increase in free-floating serotonin in the brain can trigger BDNF by binding to the 5-HT2A receptor, much like psilocybin would. It may also be an entirely new pathway that still needs to be discovered. For psychedelic scientists, this is the start of an exciting new world of possibilities.
So if all of this is true, what does it mean?
One of the front-line treatments for mental illnesses like anxiety, PTSD, and OCD is cognitive behavioral therapy (CBT), which is based on the same principles that the researchers are investigating with the classical conditioning studies. Anyone who’s participated in a session of CBT knows that the premise is actually very simple, and very reminiscent of the kind of mechanisms scientists use to train rats. Imagine you have PTSD with a specific trigger that sends you into panic attacks. A CBT approach to treatment might be exposure therapy: in a safe setting, guided by a therapist, you are gently exposed to your triggers, again and again. As time goes on and nothing terrible happens, your brain learns something new; the trigger isn’t dangerous, and slowly, your original response is made extinct. Similar techniques are used for patients with OCD (who might be unable to stop doing a particular ritual because they’re afraid something terrible will happen) and anxiety disorders. CBT is also used to treat depression, and while the mechanisms are a little different, the same basic principles apply.
It’s easy to see why, if it’s true, psychedelic neurogenesis might be useful. If we could use psychedelics to bolster and enhance our own innate capacity for learning, the applications for treatments and therapies would be tremendous. Humans are fundamentally pattern-making machines. Our most impressive technologies stem from our ability to recognize and make patterns, while some of our deepest illnesses, such as drug addiction, OCD, and depression are the result of getting caught in patterns we cannot control. If the theory laid out here is correct (and it may be entirely wrong), this could be another foundational piece on which to build psychedelic therapy. Beyond knowing just that it works, this might give us a robust, scientific understanding of why and how. It also may help us design new paradigms of psychedelic treatment: currently, almost all of the big studies being done are investigating the effects of single, medium-to-high doses of something like psilocybin, but, if psychedelic neurogenesis is real, there may be just as much therapeutic power in a series of repeated lower doses, in the right context. A medical microdose. The possibilities are endless.
Of course, such simplistic solutions should always be taken with a grain of salt: if someone tells you they have an easy-to-digest answer to a problem involving the brain, they’re probably trying to sell you something (keep that quote in mind next time someone tells you that depression is just a lack of serotonin!). Simple theories can ultimately be built into far more complete, and complex pictures, and even if this isn’t the whole picture (which it almost certainly isn’t), it seems like a pretty good place to start.
https://www.psymposia.com/magazine/do-psychedelics-trigger-neurogenesis-heres-what-we-know/