mnightchlongadong
Greenlighter
- Joined
- Apr 14, 2015
- Messages
- 2
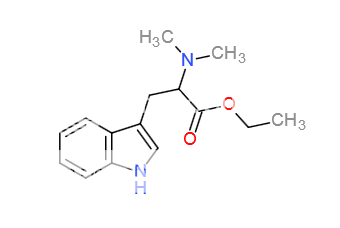
I just had this idea and came straight to the forum to see if it had been brought up before. A few years ago someone started a thread on N,N-dimethyltryptophan and its efficacy, and the idea was largely shot down, with comments regarding the polarity of the carboxyl group and subsequent degradation by MAO. My idea though is, what about an alkyl ester version of this?
To address the critiques to the former OP's idea, 1) polarity would not pose an issue is the carboxyl group were alkylated, preferably, I would think, with an ethyl or isopropyl ester, though all sorts of experimentation could be carried out here. The compound would no longer be a free acid and would in fact be highly lipophilic I would think.
Others in the old thread said that yes, the carboxyl would be removed enzymatically as with tryptophan of 5-OH tryptophan, but the drug would not be concentrated in the brain at that point due to the aforementioned polarity problem. Spread throughout the body and being rapidly metabolized by MAO would have an attenuating effect on the compound's effects, but if the alpha-carboxyl group were "esterized", I think the increase in lipophilicity would deliver much more of the compound across the BBB, where subsequent decarboxylation to DMT would take place. In essence, an orally active form of DMT with no need for MAOI coadministration. I think this could even be taken the extent of replacing the DMT portion with 4-AcO-DMT (4-acetylpsilocin) for even more lipophilicity, akin to the benefits of heroin over morphine.
I'd post pics of this but I can't figure out how. Then again, anyone in this forum already can see the tryptamine molecule in his/her head
To address the critiques to the former OP's idea, 1) polarity would not pose an issue is the carboxyl group were alkylated, preferably, I would think, with an ethyl or isopropyl ester, though all sorts of experimentation could be carried out here. The compound would no longer be a free acid and would in fact be highly lipophilic I would think.
Others in the old thread said that yes, the carboxyl would be removed enzymatically as with tryptophan of 5-OH tryptophan, but the drug would not be concentrated in the brain at that point due to the aforementioned polarity problem. Spread throughout the body and being rapidly metabolized by MAO would have an attenuating effect on the compound's effects, but if the alpha-carboxyl group were "esterized", I think the increase in lipophilicity would deliver much more of the compound across the BBB, where subsequent decarboxylation to DMT would take place. In essence, an orally active form of DMT with no need for MAOI coadministration. I think this could even be taken the extent of replacing the DMT portion with 4-AcO-DMT (4-acetylpsilocin) for even more lipophilicity, akin to the benefits of heroin over morphine.
I'd post pics of this but I can't figure out how. Then again, anyone in this forum already can see the tryptamine molecule in his/her head