sodiumglow
Bluelighter
- Joined
- Feb 9, 2006
- Messages
- 75
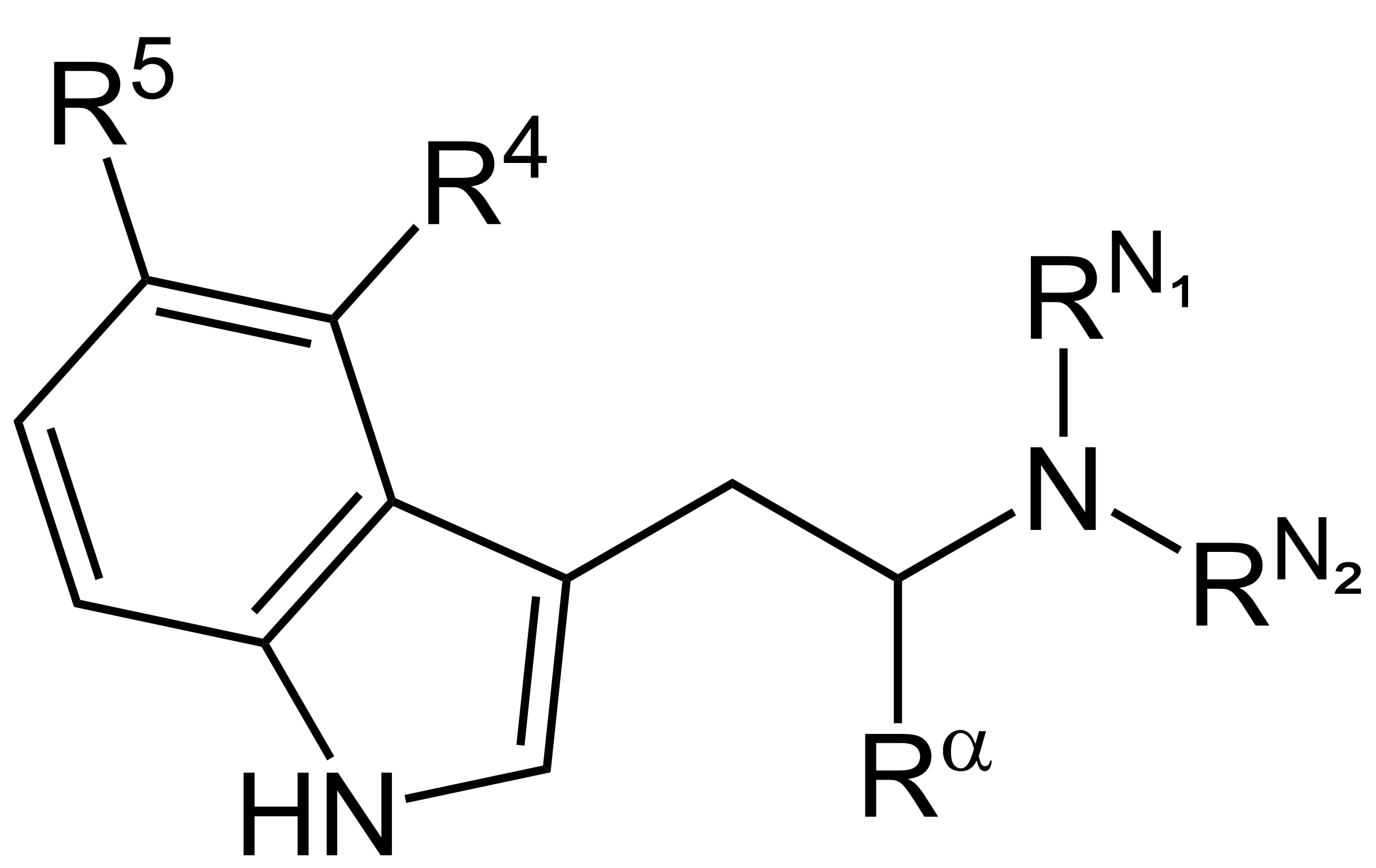
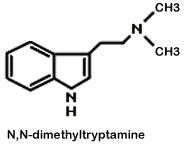
While pondering the fact that AMT is the Tryptamine homologue of Amphetamine, the idea of N,N-dimethyl-phenethylamine, the PEA homologue of DMT, popped into my head. A quick search turned up nothing that seemed to provide me with what I'm looking for. Is this compound active? Is it around? Has this thread been done x number of times? ...and if so, mods, please feel free to delete/merge.
Just curious about this I guess, and also, what about the amphetamine analogue, N,N-Dimethyl-Amphetamine? And to go a bit further (and a tad bit off course), why do some substitution patterns seem to be exclusive to the two structures? Why do para halogenated substitutions appear mostly on PEA's, and on Tryptamines you see things like 4 position Phosphorloxy and Acetoxy substitutions, and more mono and di substitutions on the amine Nitrogen?
I apologize if this has all been said and done before, but this all popped into my head as of two minutes ago while I was perusing the site and figured this would be the best place to ask, Thanks!
Just curious about this I guess, and also, what about the amphetamine analogue, N,N-Dimethyl-Amphetamine? And to go a bit further (and a tad bit off course), why do some substitution patterns seem to be exclusive to the two structures? Why do para halogenated substitutions appear mostly on PEA's, and on Tryptamines you see things like 4 position Phosphorloxy and Acetoxy substitutions, and more mono and di substitutions on the amine Nitrogen?
I apologize if this has all been said and done before, but this all popped into my head as of two minutes ago while I was perusing the site and figured this would be the best place to ask, Thanks!