Well
this graph shows LSD's affinities if you want to compare...
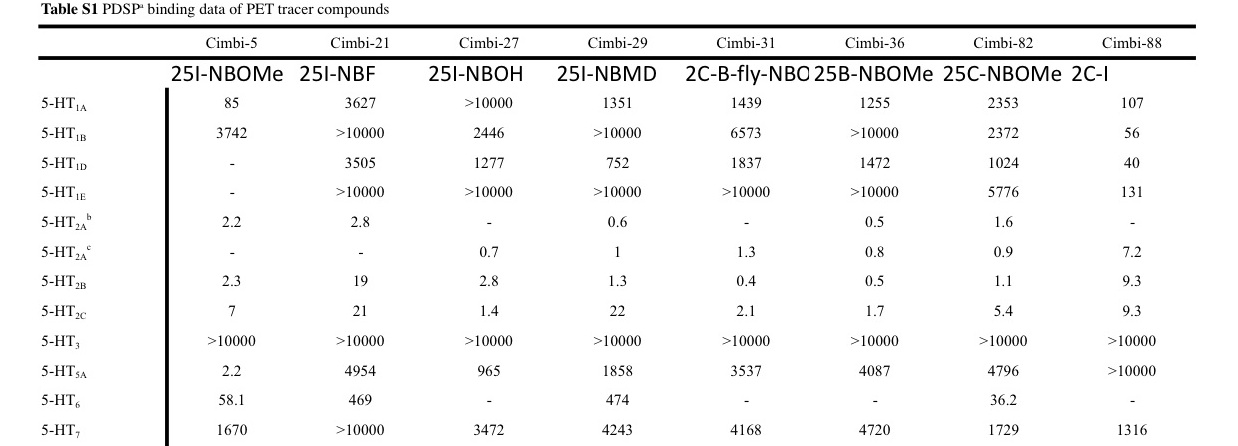
There are a few receptor binding affinities to note here, they involve the following subtypes of 5-HT (serotonin) receptor:
5-HT1A: Involved with many functions, agonists of this receptor seem to have mostly positive effects on
anxiety and depression, and they
lower heart rate and blood pressure rather than increasing them, so not immediately worrisome.
5-HT2A: Functions involved:
neuronal excitation, behavioural effects, learning, anxiety Responsible for psychedelic effects the most (significant agonism is necessary for the effect of classic psychedelics). Note that vasoconstriction is also partially regulated by it.
5-HT2B: Functions involved:
behavioural effects, vascular: pulmonary vasoconstriction,
Cardiac: The 5-HT2B receptor regulates cardiac structure.
5-HT2C: 5-HT2C receptors significantly regulate
mood, anxiety, feeding, and reproductive behavior.
5-HT5A: May have
vasodilatory and vasoconstrictive effects among other things. Probably of less importance here.
LSD at typical doses significantly affects 1A, 2A, 2C and 5A but much 2B less so.
These new results show that 25I-NBOMe may significantly affect 2A, 2B, 5A and - moderately - 2C but 1A less so.
Considering there have been deaths from 25I-NBOMe and possibly other 25X-NBOMe compounds at doses that were not even that insane, we may ask ourselves if the victims had unusually high densities of a certain receptor for which these NBOMe compounds have enough affinity and efficacy to kill, possibly mediated by cardiac and (vaso)pulmonary complications.
If we compare these values, we see that 2B is the receptor subtype that could make a difference. It is involved with functions that may be related to the complications and it could explain why NBOMe compounds can be dangerous if especially if you are unlucky (with 5-HT2B densities for example), while LSD is relatively a very safe drug.
Not only may these NBOMe compounds have the affinity to affect 2B significantly but if they also have an efficacy that LSD lacks then this could explain why LSD does not even tend to be lethal at incredibly high doses at which even LSD binds to 2B significantly. Because even if it binds at that point, lack of efficacy would still mean a lack of activation that would otherwise lead to potentially dangerous complications.
However it seems 2B issues would mostly promote chronic toxicity and not acute toxicity.
A lot of this is speculation, as we do not know the efficacies as is already mentioned, and there may be far more complex pharmacodynamics going on. LSD is -IIRC - unusual because it sets in motion a signal cascade that causes the trip, even though levels of LSD dwindle from breakdown. Maybe NBOMe compounds have similar qualities to LSD regarding the way they affect receptors but they might be more persistent and this could be dangerous on your system. I might be talking out of my ass and I might not even be the ADDer to ask these questions to begin with, but we might be onto something.
Hopefully this offers a nuanced explanation of how these drugs are 'harsh' with regard to how strongly they bind to receptor subtypes. I expect others to step in if I made mistakes or oversimplifications that give you the wrong impression.