aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
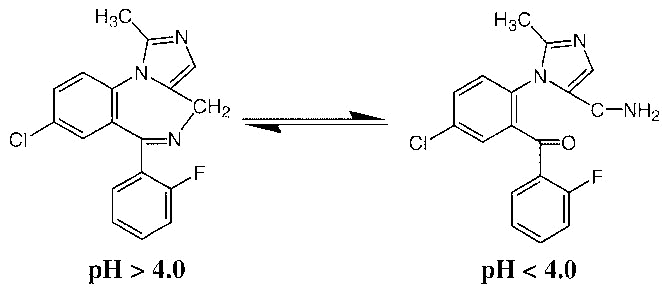
Why is midazolam soluble in water? Why is, say, diazepam not?
Midazolam has 3 nitrogens where hydrogen bonds could form? Diazepam has 2 nitrogen and also a ketone group. So would one not expect similar solubilities? Diazepam is only soluble in solvents like propylene glycol, but midazolam is soluble in water, and is appropriate for IV usage.
Also just realised that the extra nitrogen in midazolam has its lone pair electrons delocalised around the ring structure, making it less able to form a hydrogen bond. Why is this compound soluble in water?
Midazolam has 3 nitrogens where hydrogen bonds could form? Diazepam has 2 nitrogen and also a ketone group. So would one not expect similar solubilities? Diazepam is only soluble in solvents like propylene glycol, but midazolam is soluble in water, and is appropriate for IV usage.
Also just realised that the extra nitrogen in midazolam has its lone pair electrons delocalised around the ring structure, making it less able to form a hydrogen bond. Why is this compound soluble in water?
Last edited: