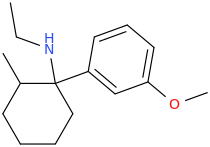
It will be very similar to MXE. The QSAR is detailed in the EJoMC article I referenced. Of course, their are 4 enantiomers and I think only 1 is active. Of course, the LogP will be improved so maybe it's twice as active? Still, that still leaves 3 unwanted compounds that may have SOME effect.
The problem is that all of the arylcyclohexylamine derivatives are controlled.
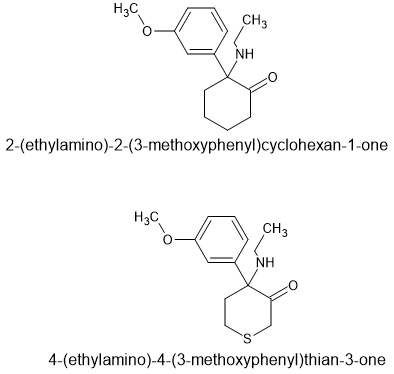
That is why simply substituting a 4 thione for the cyclohexyl moiety is the most appropriate - it allows one to retrace the substitution patterns used in all of those novel RCs thus far marketed.
Image ana hosted in ImgBB

ibb.co
Read the latest articles of European Journal of Medicinal Chemistry at ScienceDirect.com, Elsevier’s leading platform of peer-reviewed scholarly literature

www.sciencedirect.com
Now, the APPROPRIATE analogue with a 2-thiophene aromatic and N-ethyl amine has sub nM affinity for the NMDA receptor but I always have to remind people that what K and all of the popular RC agents have NMDA antagonist, mu agonist & DRI activity. It's finding the balance that is key. Something that is just a highly potent NMDA antagonist may well have a market, but it wouldn't be as 'good' as MXE.
As far as I can see, swapping the cyclohexyl for a 4-thiane is going to be the most likely to directly substitute. Yes, the 4-thianes had more NMDA activity than the cyclohexyls but it wasn't an order of magnitude, it was about 10% if memory serves.
Of course, the chemistry behind such a substitution is interesting. But that is always the case. People are keen to name ligands with no way of making them which isn't practical.