I was also curious about the isomer that was typically available. I'm fairly certain the majority of what is being sold is d-threo-EPH. There were reports years back about l-threo-EPH available, and that this was far weaker, subjectively. I'd have to look into the synthesis routes that they would use, but I believe that the synthesis does produce the threo diastereomer, and not the erythro.
A simple polarimeter could be used to test if it's d,l-EPH (zero rotation) or d-EPH (+ rotation). If anyone has a sample, they can do a test using a LCD monitor, polarized sunglasses, DI water, and sugar for reference. I would suspect that people would notice an approximately halved potency using the d,l-threo product compared to the d-threo and this would be reported. I'd also expect the d-threo would tend to crystallize out in larger crystals, which is consistent with some reports on appearance.
As far as the other, inactive, isomers giving rise to undesirable drug side effects I have doubts about that. While d-threo-MPH has been available as a drug, the exact difference in effects produced form the d,l is still not clear. FWIW, I could not tell the difference after accounting for potency differences. The potency of EPH relative to pharmaceutical d,l-threo methylphenidate is largely consistent between literature (binding studies give a clue) and subjective reports, which also suggests to me that it is not contaminated with other isomers.
An aside, since we're talking a bit about EPH chemistry: A major problem about EPH-HCl compared to MPH-HCl is that the ethyl ester in EPH appears to be much easier to hydrolyze than the methyl ester in MPH. Thus, ritalinic acid is produced in significant amounts quite rapidly when consumed. This is almost surely the explanation why EPH is so dramatically caustic to nasal membranes and other tissues (relative to MPH).
ps. the [a]D20 for the active isomer of ethylphenidate is reported as +65.10 (c 1.08, methanol)
what does this mean ?
the specific rotation for d-threo-EPH in methanol. if anyone has a lab polarimeter they could measure this

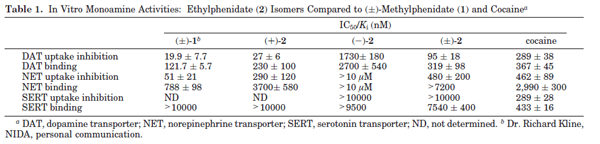
 mind boggling. Do the authors mention it all in the discussion?
mind boggling. Do the authors mention it all in the discussion?