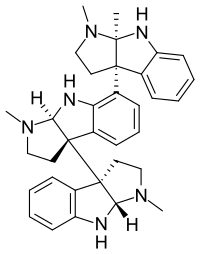
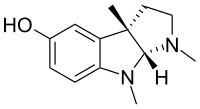
I've seen those overlay diagrams appear in several articles and books, especially when it's about something binding to the opioid receptors.
The molecular docking simulations are also an interesting topic, but it is a hassle to find the potential binding sites from the 3D receptor protein model, to set the protonation of amine and carboxyl groups to correspond to physiological pH and so on. Even though it's based on just classical electromagnetism without any quantum mechanics that would make it computationally impossible at the current status of available computational power. I hope there will soon be some type of AI tool that makes this process easier.