Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Levophacetoperane (Lidépran, Phacétoperane) is twice as potent as methylphenidate (Ritalin), it's only used in France, it's only controlled in France and it is not covered by the UNODC list of controlled drugs.
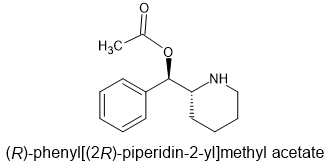 It's totally legal to obtain the immediate precursor:
It's totally legal to obtain the immediate precursor:
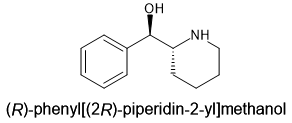
And, as for acetylation, not only acertic anhydride will work. Aceyl propanoic anhydride, ethane-1,1-diyl diacetate, 1-(acetyloxy)ethyl propanoate, propane-1,1-diyl diacetate or indeed ANY mixed anhydride or mixed diyl. When the only limits are physical (melting point and boiling point), one realises that there are about 120 differenc compounds and many of them can be made at home using a simple synthetic pathway. Benzoic acids are problematic so pyridine-3-carboxylates (isonicotinic acid derivatives) are convenient. When you esterify, the LIGHTER acid forms the ester.
So, now we have just destroyed any semblance of AA control meaning anything. I mean, I know Russian chemists who use Aspirin for transesterification - where there is a will there is a way. Oh, and patents abound, seek and thee shall find.
But what was 'hot property 10 years ago was the idea of:

When examined using ChemOffice, it looked like a winner BUT the problem was that the Reaxys reference was WRONG. We tried an Italian company who was after our work but rather than finding that the paper didn't work and finding a route that DID work, they just complained that the paper was wrong. Their loss. We spent close to £1 million on new synthetic pathways that we passed on to others for bulk synthesis.
But I am sure people can see that it is very simpler to the bk-amphetamines. Even the meanest intelligence knows that in the case of the bks, a tertiary amine is fine. Even if not, the N-benzyl is quickly removed from the body and if they had used the triflate to protect the secondary amine, all would be well. I was surprised how a company whose entire reason du jour was finding answers was so unhelpful.
Other scaffolds worked and we never got back to it BUT it's an interesting unknown. An acid halide & boronic acid would seem appropriate.
After all, the benzylpiperidines do seem to mirror the PEAs rather well with a team at Perdue discovering DMBMPP. People wondering if the aminorex scaffold needs researching, I can confirm that we tried 8 or 9 different analogues and sent Dr. Nichols the results. They were not positive. It's possible to make an empathogen, but not a 5HT2a ligand.
Dr. Nichold was very kind and answered a KEY question. Whereas the aminorex scaffold is NOT an MAOI, the 4-methyl aminorex scaffolds ARE. It only took a polite E0mail to ask and yet an unnamed Israeli chemist insisted in releasing 'Serotonia' AKA p,4-dimethylaminorex. I never met him but I never met anyone who liked him. He made a big deal out of 'discovering' 5-APB & 6-APB although the 2 compounds had already been patented! Still I guess people should not seek noble aims in RC designers.
I WAS impressed by m-F phenmetrazine, especially since a guy I worked with had 3,4-MD phenmetrazine made only to prove that it didn't work. A 2:1 mixture of m-Me/p-Me phenmetrazine will, I am quite sure, be identical to MDMA.


And, as for acetylation, not only acertic anhydride will work. Aceyl propanoic anhydride, ethane-1,1-diyl diacetate, 1-(acetyloxy)ethyl propanoate, propane-1,1-diyl diacetate or indeed ANY mixed anhydride or mixed diyl. When the only limits are physical (melting point and boiling point), one realises that there are about 120 differenc compounds and many of them can be made at home using a simple synthetic pathway. Benzoic acids are problematic so pyridine-3-carboxylates (isonicotinic acid derivatives) are convenient. When you esterify, the LIGHTER acid forms the ester.
So, now we have just destroyed any semblance of AA control meaning anything. I mean, I know Russian chemists who use Aspirin for transesterification - where there is a will there is a way. Oh, and patents abound, seek and thee shall find.
But what was 'hot property 10 years ago was the idea of:

When examined using ChemOffice, it looked like a winner BUT the problem was that the Reaxys reference was WRONG. We tried an Italian company who was after our work but rather than finding that the paper didn't work and finding a route that DID work, they just complained that the paper was wrong. Their loss. We spent close to £1 million on new synthetic pathways that we passed on to others for bulk synthesis.
But I am sure people can see that it is very simpler to the bk-amphetamines. Even the meanest intelligence knows that in the case of the bks, a tertiary amine is fine. Even if not, the N-benzyl is quickly removed from the body and if they had used the triflate to protect the secondary amine, all would be well. I was surprised how a company whose entire reason du jour was finding answers was so unhelpful.
Other scaffolds worked and we never got back to it BUT it's an interesting unknown. An acid halide & boronic acid would seem appropriate.
After all, the benzylpiperidines do seem to mirror the PEAs rather well with a team at Perdue discovering DMBMPP. People wondering if the aminorex scaffold needs researching, I can confirm that we tried 8 or 9 different analogues and sent Dr. Nichols the results. They were not positive. It's possible to make an empathogen, but not a 5HT2a ligand.
Dr. Nichold was very kind and answered a KEY question. Whereas the aminorex scaffold is NOT an MAOI, the 4-methyl aminorex scaffolds ARE. It only took a polite E0mail to ask and yet an unnamed Israeli chemist insisted in releasing 'Serotonia' AKA p,4-dimethylaminorex. I never met him but I never met anyone who liked him. He made a big deal out of 'discovering' 5-APB & 6-APB although the 2 compounds had already been patented! Still I guess people should not seek noble aims in RC designers.
I WAS impressed by m-F phenmetrazine, especially since a guy I worked with had 3,4-MD phenmetrazine made only to prove that it didn't work. A 2:1 mixture of m-Me/p-Me phenmetrazine will, I am quite sure, be identical to MDMA.