_DankOpiAmp_
Bluelighter
- Joined
- Sep 13, 2013
- Messages
- 1,036
I watched a YouTube video from reddit the other day and was wondering if it is always required. The individual in the video seemed to have perfectly double boiled and air dried it so that it barely changed after vacuum purging. The shatter was hard to stick together without heating pre- purge. It would also crack into chips/shards when scraped ( like its namesake?!)
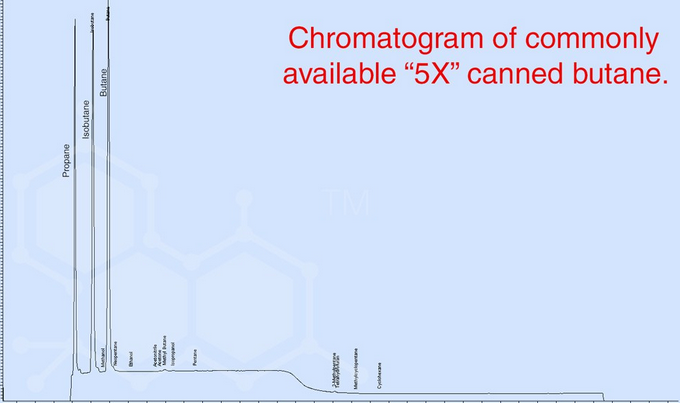
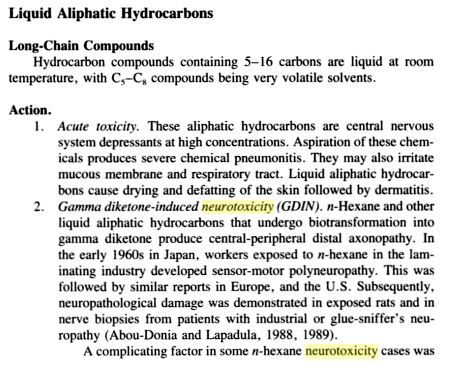
It seems that uncontaminated butane's only health risk is the displacement of oxygen (which can lead to asphyxia), but this would only be possible if you were inhaling the vapour without oxygen. Its chemical hazard sheet seems to indicate that is is not "toxic" in the traditional sense and the properly boiled/aired out shatter seemed to have minimal butane residue anyway.
The collection Pyrex dish was quite large for the yield so the increased surface area from being so thinned out may have allowed more butane to evapourate. The shatter had be double boiled for an hour then left of to dry for 14 hours.
It seems that uncontaminated butane's only health risk is the displacement of oxygen (which can lead to asphyxia), but this would only be possible if you were inhaling the vapour without oxygen. Its chemical hazard sheet seems to indicate that is is not "toxic" in the traditional sense and the properly boiled/aired out shatter seemed to have minimal butane residue anyway.
The collection Pyrex dish was quite large for the yield so the increased surface area from being so thinned out may have allowed more butane to evapourate. The shatter had be double boiled for an hour then left of to dry for 14 hours.