-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Is methoxetamine chiral?
- Thread starter MyExcuse
- Start date
Iodjini_dk
Bluelighter
- Joined
- Sep 16, 2004
- Messages
- 344
Yes, it contains one stereocenter. Thus it exists as two enantiomers. Commonly available methoxetamine is a racemic mixture as far as Im aware.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Maybe take a look at the Ketamine Isomers Subthread in PD (via navigation of: the Psychedelic Index >> Ketamine), then extrapolate what you read there to methoxetamine and take a guess at which isomer does the most reuptake inhibiting or which one would be more purely dissociative. It may turn out that even classical dissociation is comprised of more than one component (cognitive, proprioceptive, existential, ...)
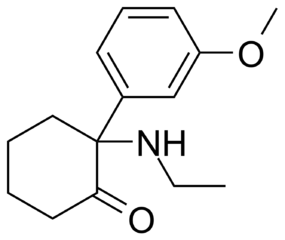
The chiral carbon is the one represented by the vertex (point / corner) immediately left of the N(itrogen). Why? Because 4 things attached to that one are NOT interexchangable. Which makes it an asymmetrical locus.
I wonder... is all it takes to separate MXE isomers precipitation with a pure tartaric acid enantiomer?

The chiral carbon is the one represented by the vertex (point / corner) immediately left of the N(itrogen). Why? Because 4 things attached to that one are NOT interexchangable. Which makes it an asymmetrical locus.
I wonder... is all it takes to separate MXE isomers precipitation with a pure tartaric acid enantiomer?
Last edited:
Transform
Bluelight Crew
- Joined
- Sep 5, 2010
- Messages
- 4,791
That's how I did it.
I only tried one isomer so far, and the procedure was very imprecise, but it seemed considerably less potent than normal.
This was accounting for the fact it was the tartarate and contained precipitated NaCl so it seems like it was quite successful.
The procedure was quite a lot of hassle and I've not tried it again since because I enjoyed MXE so much on its own.
NB the synthesis of MXE produces a perfect 50/50 mix so this phenomenon cannot be used to explain the low potency of your batch of MXE, dear reader.
I only tried one isomer so far, and the procedure was very imprecise, but it seemed considerably less potent than normal.
This was accounting for the fact it was the tartarate and contained precipitated NaCl so it seems like it was quite successful.
The procedure was quite a lot of hassle and I've not tried it again since because I enjoyed MXE so much on its own.
NB the synthesis of MXE produces a perfect 50/50 mix so this phenomenon cannot be used to explain the low potency of your batch of MXE, dear reader.
I am sorry for opening this thread again, but I have just come across this discussion and would like to ask @Transform if you have been able to successfully separate the enantiomers of Methoxetamine. If so, which chiral derivatisation agent did you use, if I may ask?
I haven't found any publication about chiral separation of MXE using LC- or GC-MS. Does anyone know of any?
I haven't found any publication about chiral separation of MXE using LC- or GC-MS. Does anyone know of any?
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Pretty sure the answer to that is L-(+)-tartaric acid which is also used a lot in brewing / winemaking. It is a substance used for separating isomers of various compounds, also like amphetamine for example.
As long as there is a significant difference in how (energy efficiently) this tartaric acid isomer forms salts with the isomers of a drug, you should see that as a difference in tendency to crystallize... allowing you to crystallize one isomer of the drug first and the other one after that, after as in under changed conditions like cooled or evaporated or further addition of a second solvent.
Depending on how big that difference is though, and how well you are able to use the correct solvent systems etc... achieving that separate crystallization may range from doable to quite difficult.
What I don't really know is which MXE isomer would crystallize with L-(+)-tartaric acid, I suspect that it's the levo with the levo for point-symmetry.
You don't use LC or GC-MS for chiral separation I don't think, as the physical properties involved with that are not different for the isomers? A polarimeter though can tell you the optical activity.
EDIT: oh the dextro was used... well I guess same story applies, only the levo should be more available?
I wonder, was there a marked gap between when one isomer crystallized and stopped crystallizing, and then the other under changed conditions? Or was it more continuous? If continuous, I guess it would be very hard to really be useful.
Anyway.... with most drugs who would be bothered to isolate the solely active isomer?... but with something like amphetamine or certain dissociatives where the other isomer also has activity but different... then you more or less just have two different drugs that may be worth experiencing by themselves! Something possibly overlooked with analogues of these substances, to be fair we probably often just don't know about the different activity with novel analogues.
As long as there is a significant difference in how (energy efficiently) this tartaric acid isomer forms salts with the isomers of a drug, you should see that as a difference in tendency to crystallize... allowing you to crystallize one isomer of the drug first and the other one after that, after as in under changed conditions like cooled or evaporated or further addition of a second solvent.
Depending on how big that difference is though, and how well you are able to use the correct solvent systems etc... achieving that separate crystallization may range from doable to quite difficult.
What I don't really know is which MXE isomer would crystallize with L-(+)-tartaric acid, I suspect that it's the levo with the levo for point-symmetry.
You don't use LC or GC-MS for chiral separation I don't think, as the physical properties involved with that are not different for the isomers? A polarimeter though can tell you the optical activity.
EDIT: oh the dextro was used... well I guess same story applies, only the levo should be more available?
I wonder, was there a marked gap between when one isomer crystallized and stopped crystallizing, and then the other under changed conditions? Or was it more continuous? If continuous, I guess it would be very hard to really be useful.
Anyway.... with most drugs who would be bothered to isolate the solely active isomer?... but with something like amphetamine or certain dissociatives where the other isomer also has activity but different... then you more or less just have two different drugs that may be worth experiencing by themselves! Something possibly overlooked with analogues of these substances, to be fair we probably often just don't know about the different activity with novel analogues.
Last edited:
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
Chiral HPLC can be used to seperate the enantiomers of ketamine:
http://www.tandfonline.com/doi/pdf/10.1080/01496390500283258
http://www.tandfonline.com/doi/pdf/10.1080/01496390500283258
