Cool fact, but not really relevant to the topic at hand.
It appears that Dave Nichols has only ever made bromo-DragonFLY. Looking at
the synthesis, it would not be terribly hard to substitute I
2 for the Br
2 used to install the halogen. (Or, alternatively, some sort of chlorinating/fluorinating/trifluoromethylating agent for the respective analogues.) Alkyl analogues would probably need different synthetic methods.
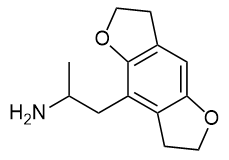
For those who really want to know: The synthesis actually begins with DOH-FLY (1-(2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)propan-2-amine, SMILES: CC(N)CC1=C2C(OCC2)=CC3=C1OCC3 ), the nitrogen is protected (as a trifluoroacetate), then it is brominated with Br
2 (forming N-TFA-DOB-FLY), the "wings" are oxidised with
DDQ (forming N-TFA-Bromo-DragonFLY), and finally the nitrogen deprotected with base forming Bromo-DragonFLY as a freebase, which was added to ethanolic HCl and diluted with ether, forming the final product: Bromo-DragonFLY HCl.
If you just avoid the DDQ you could make much safer and less superpotent analogues. (Bromo-DragonFLY is a MAOI as well as a 5HT2A agonist)