Megalum
Bluelighter
- Joined
- Jul 10, 2010
- Messages
- 101
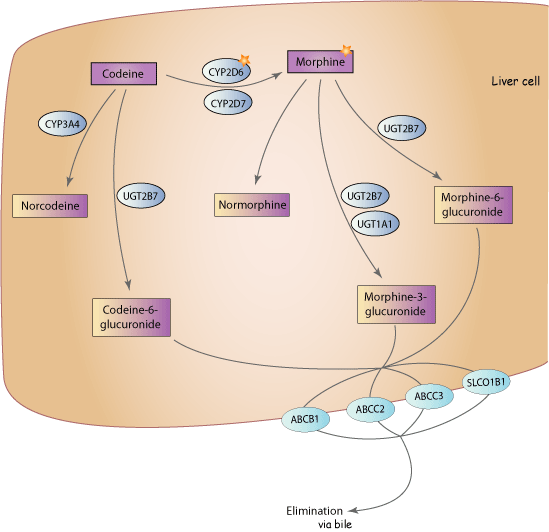
*source https://www.pharmgkb.org/pathway/PA146123006#
The studies so far show mixed results on UGT2B7 effectiveness. So yes, this is a probably a pipe-dream thought... but, you know... drugs.
I know everyone would love a successful means to have more Codeine -> Morphine conversion rate higher than the avg 10%~
People have looked ALL through CYP2D6 and there isn't much there as of yet. Theoretically, inhibiting UGT2B7 could have some real potential.
For one, it would slow the conversion of the relatively useless codeine-6-glucuronide,
UGT2B7 catalyzed morphine glucuronidation at the 3- and 6-hydroxy positions and also mediated the formation of codeine-6-glucuronide from codeine.
*sourcehttps://www.pharmgkb.org/pmid/9010622
Now, I realize this would also lower the amount of the 3 and 6 forms of metabolized morphine, but that seems a small sacrifice for the extra CYP2D6 pathway being forced to metabolize more codeine.
That being said, I need help researching a list of possible candidates to inhibit UGT2B7 -- the most likely source I've seen so far is Diclofenac, here is some data regarding the aforementioned.
http://www.cyprotex.com/useruploads/UGT-inhibition-fig3-5.png
For record, they used Naloxone as the substrate to test diclofenac's potency with regard to inhibitory effect.
Any comments, discussion, please help me crack the code
