-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,885
I want to mention that anyone taking the results of STP as gospel truth of binding, rather than a wild-ass guess, needs to have their head looked at
I'm not anywhere near that stupid. The most important feature of the STP is that it tells you if something is known to definitely bind to some receptor.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Does ascorbic amphetamine exist? Does the salt's structure modify its pharmacology? In the way that I actually never understood if the drugs like meth turned to a base in the blood or if the bloods dynamics permitted the drug to remain a salt eventhough in "solution". For example some drugs tend to cause disruption to the blood if IV'd, thus first made into a base and an acid as a real "solution", like with coke giving orc blood, and not if absorbed through the mucosa, where the surface is humid but ionised differently than water. Sekio?
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,993
"amphetaminium hydroxide" would be unstable and would decompose to water + freebase amphetamine.
the ascorbate salt of amphetamine is also known but the problem is that the counterion does not really matter - given the enviroment of the body where there is chloride, sulfate, acetate etc the counterions will swap around. so no, salts usually do not influence the pharmacology only the pharmacokinetics (how fast they dissolve etc)
at ph 7 most of the common drugs exist as the free base in the blood, some solvated in plasma directly but most either binding to plasma protiens or fatty cell membranes

Einsteinium-253 in a quartz vial glowing due to its radioactivity. Cool shit.
the ascorbate salt of amphetamine is also known but the problem is that the counterion does not really matter - given the enviroment of the body where there is chloride, sulfate, acetate etc the counterions will swap around. so no, salts usually do not influence the pharmacology only the pharmacokinetics (how fast they dissolve etc)
at ph 7 most of the common drugs exist as the free base in the blood, some solvated in plasma directly but most either binding to plasma protiens or fatty cell membranes

Einsteinium-253 in a quartz vial glowing due to its radioactivity. Cool shit.
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Cleared my mind. Thanks
Edit: putting my posts together.
Sekio have you tried synthing "blæzøcaïne"? The COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C
Should be UK legal
Blaezocaine or "blowsallcaine",
I think the way to go is beta-alanine, ketopyridine and benzoic acid.
Pure dopa: http://swisstargetprediction.ch/result.php?job=642486740&organism=Homo_sapiens
Look at the known actives! http://www.swisstargetprediction.ch/result.php?job=1641719302&organism=Homo_sapiens
Edit: putting my posts together.
Sekio have you tried synthing "blæzøcaïne"? The COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C
Should be UK legal
Blaezocaine or "blowsallcaine",
I think the way to go is beta-alanine, ketopyridine and benzoic acid.
Pure dopa: http://swisstargetprediction.ch/result.php?job=642486740&organism=Homo_sapiens
Look at the known actives! http://www.swisstargetprediction.ch/result.php?job=1641719302&organism=Homo_sapiens
Last edited:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,993

dissociatives

Synthesis and Pharmacological Properties of 1-(6-Aminohexylamino)-1-Phenylcyclohexyl Dihydrochloride (IEM-2062) as Compared with Memantine - Pharmaceutical Chemistry Journal
1-(6-Aminohexylamino)-1-phenylcyclohexyl dihydrochloride (IEM-2062) had significantly greater antihypoxic, anticonvulsant, antidepressant, and analgesic activity than memantine and similar antiparkinsonism activity as memantine; it had low toxicity and was safer for use. IEM-2062 produced...

Synthesis and biological activity of phencyclidine and its adamantylamine derivatives
1-(2-Phenyl-2-adamantyl)amines 2a-c were synthesized and their biological activity evaluated by in vitro testing of their effects on the proliferative…

G. Lenz says this has a longer duration and is more potent than pethidine. never would have thought of an adamantanol ester
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Reposting:
Here I'll be posting my best inventions, selected for their binding profiles.
It'll take some time to finish this thread (if I ever do as I've litterally thousands of chemicals to sort out and run through, and test with the SAR machine), but I'll post what I think deserves to be posted.
We'll start with Ketamine and Ephenidine analogs I've invented, and switch to BTCP analogs which will permit us to switch to DRIs: (And from there it'll be pretty random, as I can't classify all my compounds)
Ketamine
PCP
Ephenidine
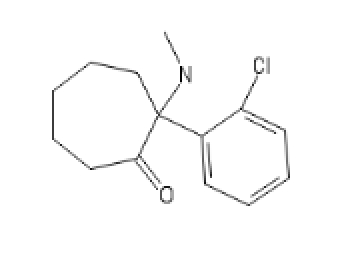
Seven ringed Ketamine,
CycloHeptylKetamine : C1(C(CCCCC1)=O)(C2=CC=CC=C2)N(C)[H]
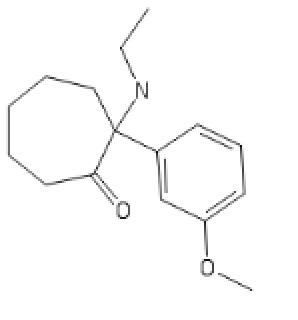
Seven ringed Methoxetamine,
CycloHeptylMethoxetamine: C1C(C(CCCC1)(C2=CC=CC(=C2)OC)NCC)=O
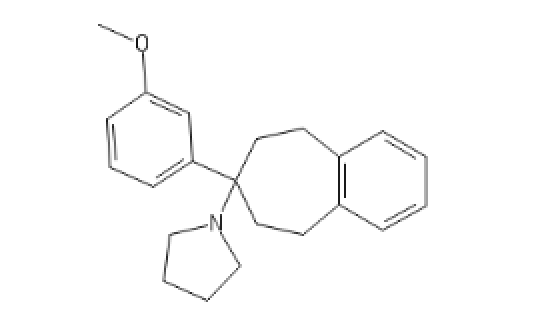
Adding a benzene group to the cycloheptyl:
3-MeO-PCPy with a PhenylCycloHEptyl ring: C1CC(CCC2=C1C=CC=C2)(C3=CC=CC(=C3)OC)N4CCCC4
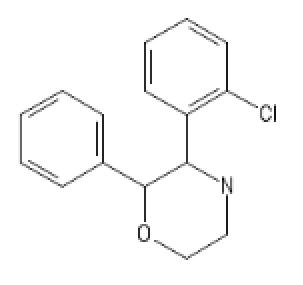
a little extra
(With very interesting SARs)
MorphoKetamine: ClC1=C(C=CC=C1)C1NCCOC1C1=CC=CC=C1
PCP with a N-PhenylPiperazine
PCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C4=CC=CC=C4
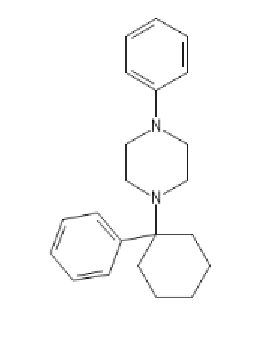
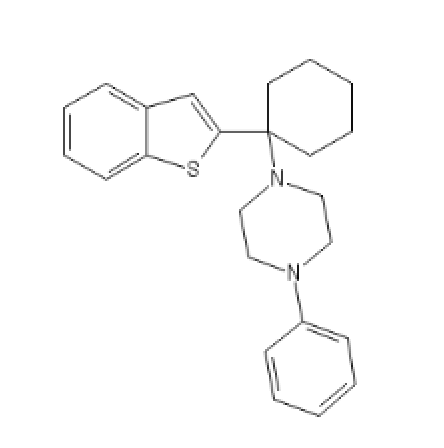
BTCPP, BTCP with a phenylpiperazine
BTCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C5=CC4=CC=CC=C4S5
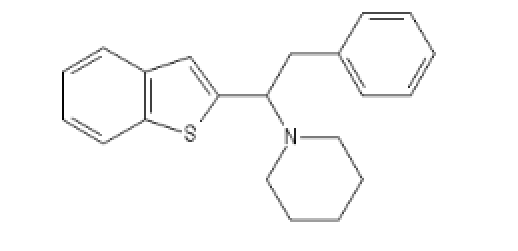
Three analogs between Ephenidine and BTCP:
BTPhenEthylPiperidine : C(C(N1CCCCC1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
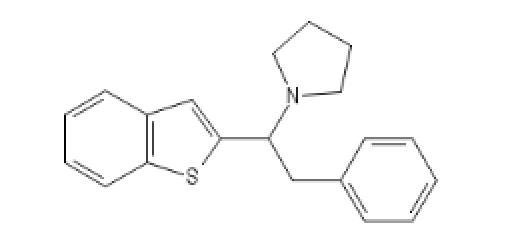
BTPhenEthylPyrrolidine: C12=C(C=CC=C1)SC(=C2)C(CC3=CC=CC=C3)N4CCCC4
BTBKPhenEthylEthylamine: C12=C(C=CC=C1)SC(=C2)C(C(C3=CC=CC=C3)=O)N(CC)[H]
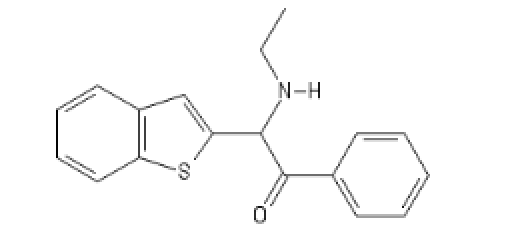
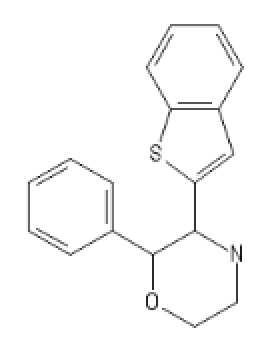
The morpholine analog of ketamine applied to BTCP:
MorphoBTCE: C1COC(C(N1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
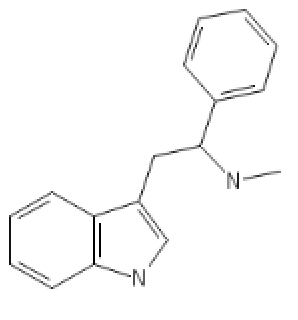
Mono-methylated alpha-phenylated tryptamine, a SNDRI that stands out:
N-Methyl-Alpha-Phenyl-Tryptamine: C1=CC=CC2=C1C(=C[N]2)CC(C3=CC=CC=C3)NC
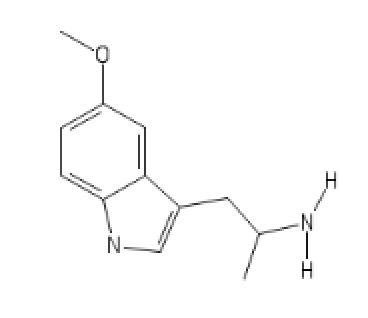
Its N-Pyrrolidino analog
5-MeO-AMT
C1(=CC=C2C(=C1)C(=C[N]2)CC(N([H])[H])C)O
Cocaïne analogs: probably my best inventions
I Don't know what to call these, but they're wonderously promising
COC(=O)C(CC1=CC=CC=C1)C1CCCN1
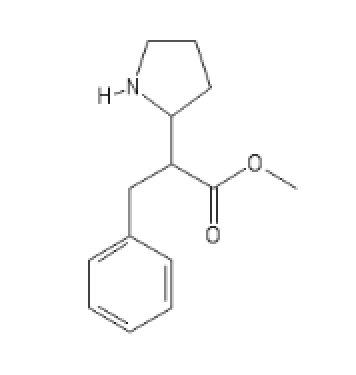
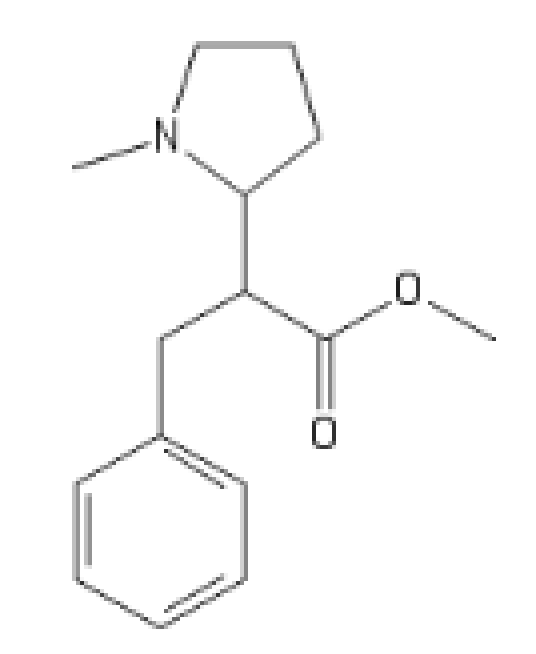
The N-Methyl, even more promising
C1(=CC=CC=C1)CC(C(=O)OC)C2CCCN2C
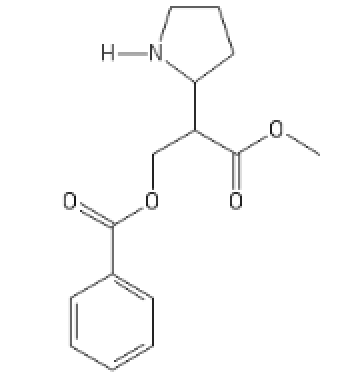
The local anaesthetic version, it's got high HERG activity:
C1=CC=CC=C1C(OCC(C(OC)=O)C2CCCN2[H])=O
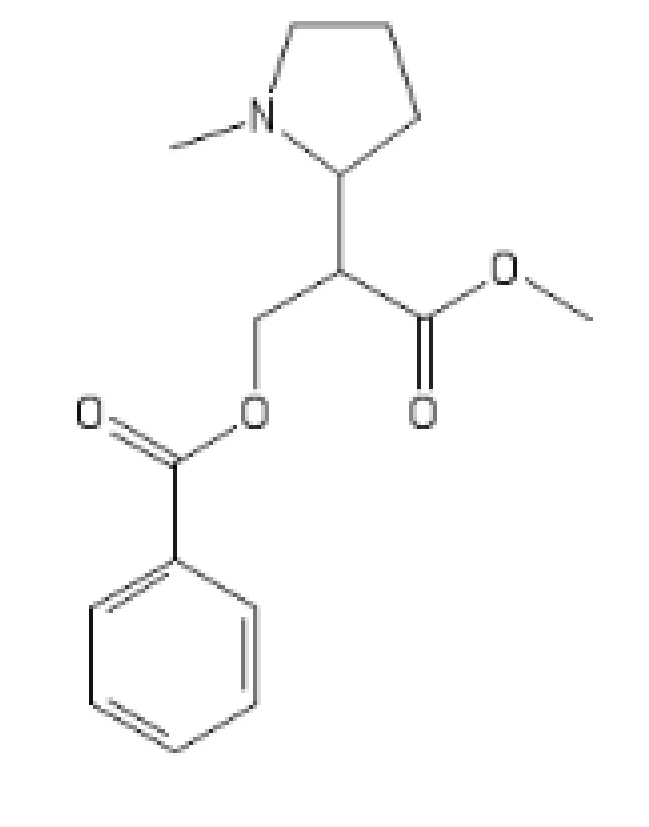
The N-Methyl version, with even more HERG activity, which makes it cardiotoxic
COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C
An outstanding one,both SNDRI and MOR (!)
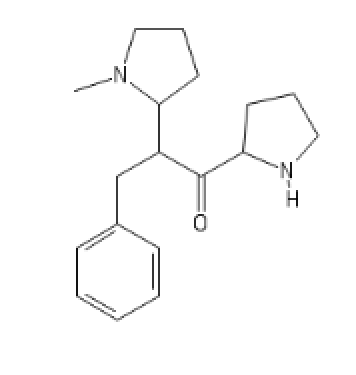
N'-N-Methyl version
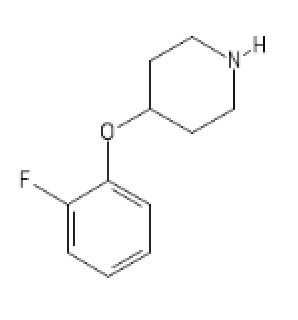
2-FA Light
C1(=CC=CC=C1OC2CCN(CC2)[H])F
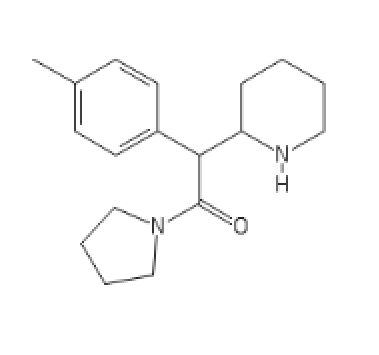
4-MethylMethylphenidate N-pyrrolidine analog
C1=CC=CC=C1C(C(N2CCCC2)=O)C3CCCCN3[H]
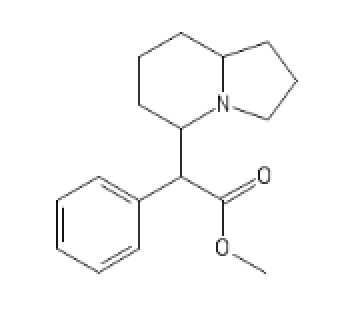
PVP Methylphenidate: Surprisingly good
C1=CC=CC=C1C(C2CCCC3CCCN23)C(OC)=O
N-pyrrolidino Version
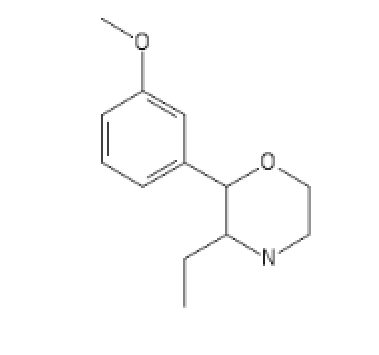
3-MeO-PhenEtrazine
C1=C(C=CC=C1C2C(CC)N(CCO2)[H])OC
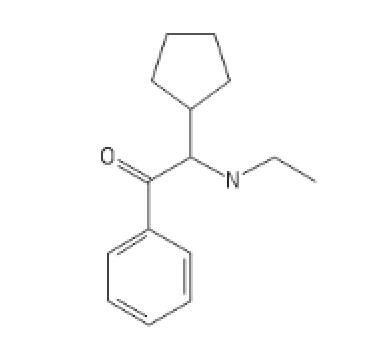
CyclopentylEthylCathinone
CCNC(C1CCCC1)C(=O)C1=CC=CC=C1
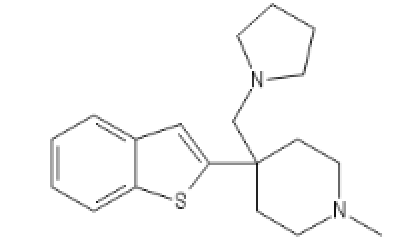
Two Ketobemidone variants, go guess how I guessed
BTKETO1
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)CN4CCCC4
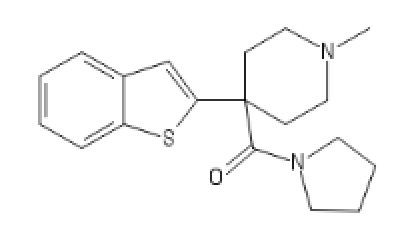
BTKETO2
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)C(N4CCCC4)=O
Should be opioïd, is Dopaminergic and Opioïd sigma active:
C2(CC(CC1=CC=CC=C1)N(CC2)[H])(C(=O)CC)C3=CC=CC=C3
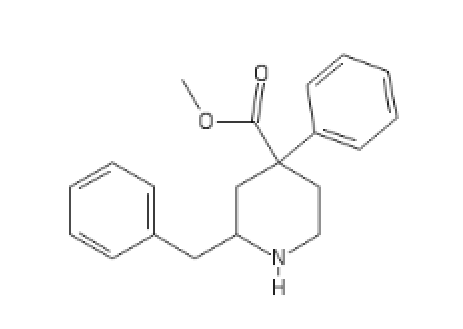
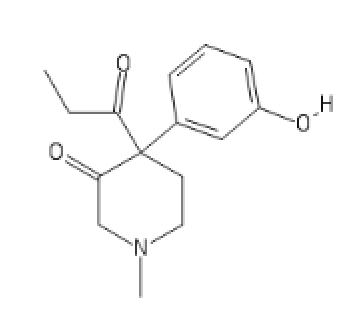
Ketobemidone analog:
C1(C(CN(CC1)C)=O)(C(=O)CC)C2=CC(=CC=C2)O[H]
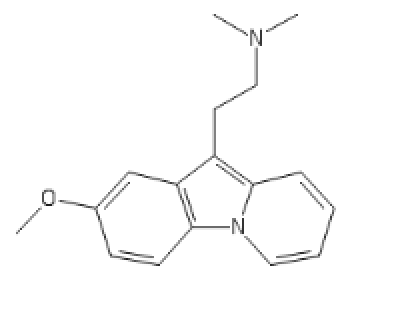
Chlorphenamine derived Antihistaminergic Psychedelics, or chillaxed tripping!
5-Meo one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)O
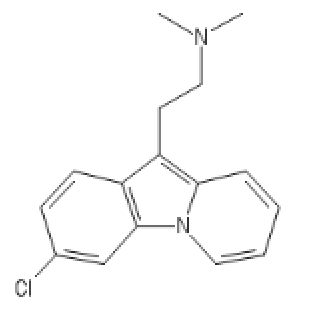
Chlorphenamine inspired
C1=CC(=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)Cl
5-Meo DIPT one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(CC=C)CC=C)OC
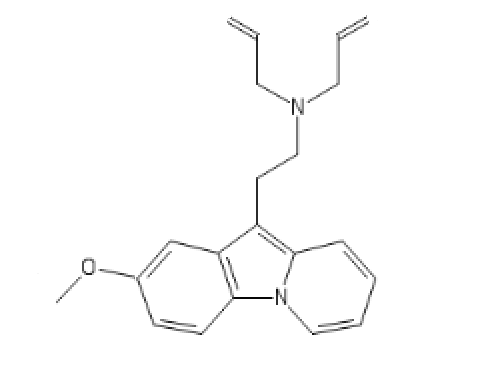
Now let's switch to killer opioïds, Fentanyl analogs and some others:
Cyclic R-30490
C1(=CC=CC=C1)CCC2CCC3(CC2)COCC(N3C4=CC=CC=C4)=O
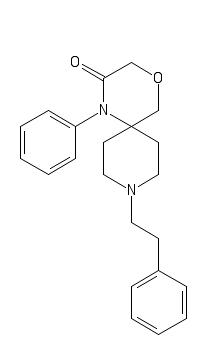
Its (probably) more potent analog, 2'-Fluoro-3-Methyl-Cyclic R-30490
C1(=CC=CC=C1)CCN2CC(C4(CC2)N(C3=C(C=CC=C3)F)C(COC4)=O)C
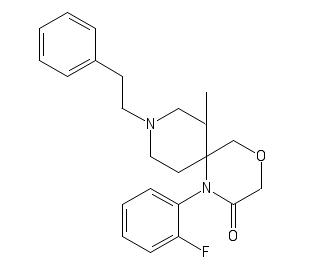
My FuranylFentanyl
C1(=CC=CC=C1)CCN2CCC(CC2)(C3=CC=CO3)N(C4=CC=CC=C4)C(CC)=O
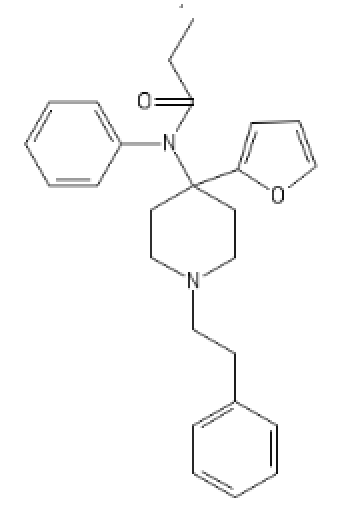
Non-Brominated Cyclic BDPC
C1=CC=C(C=C1)C2(CCC3(CC2)OCCC(C3)C4=CC=CC=C4)N(C)C
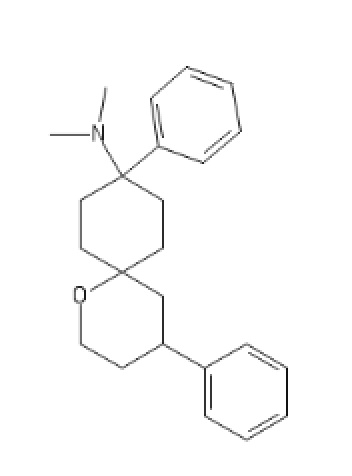
Fentanyl with the phenethyl displaced
C2(CC(CC1=CC=CC=C1)N(CC2)[H])N(C(CC)=O)C3=CC=CC=C3
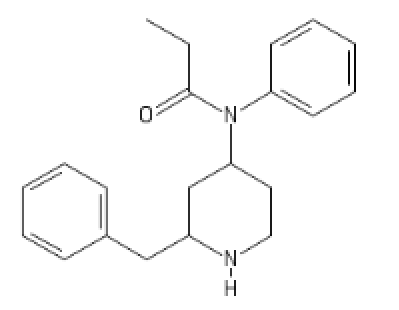
And an already seen one, but it has its place here:
An outstanding one,both SNDRI and MOR (!)

Some weird cannabinoïd, related to JWH-018
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)CC4=CC=CC=C4
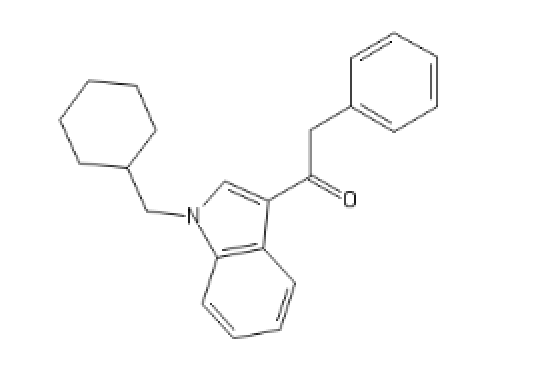
What do I call this:
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)C5C4CCCCC4CCC5
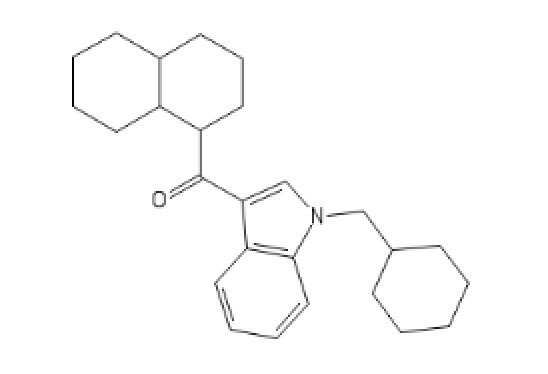
For more fun, go see ILTDPORM or Dresden's thread.
From here on the thread is dedicated to chems that aren't of my invention, mine are above ^.
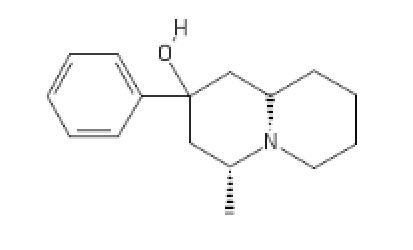
Some of polymath's inventions:
N-PE-Nor-MEthadone
Polymath's Funny little beast, Fenmyrtin:
[C@H]1(CC(CC2CCCCN12)(C3=CC=CC=C3)O[H])C
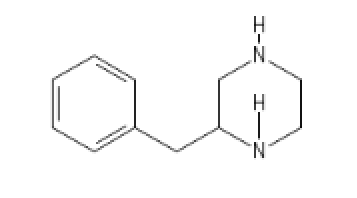
S.J.B.'s constitutional isomer of BZP
C1=CC=CC=C1CC2CN(CCN2[H])[H]
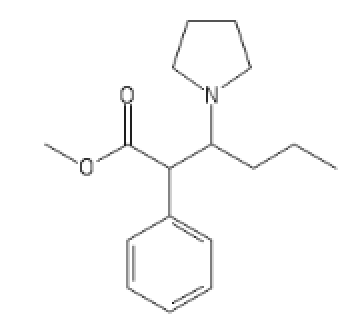
Dresden's Carbomethoxy PVP:
C1=CC=CC=C1C(C(CCC)N2CCCC2)C(=O)OC
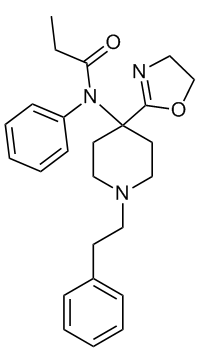
Sekio's Oxazolino Carfentanyl
C1(CCN(CC1)CCC2=CC=CC=C2)(C3=NCCO3)N(C4=CC=CC=C4)C(CC)=O
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,993
you get a pass this time because your thread was merged here, but please don't repost things 
also if you want people to be able to understand the structures you post, try either using iupac names or posting images of said compounds... to me SMILES is all greek & i have to plug every one into chemdraw to figure out what you are talking about.
also keep in mind STP is not a replacement for "real" pharmacological data you may have noticed it has some problems where it will erroneously assign strange binding sites for known ligands... BDCP is one
you may have noticed it has some problems where it will erroneously assign strange binding sites for known ligands... BDCP is one 
but in the interest of other cool tools, there is also supposedly a bioisostere replacement tool as well as a ADME prediction tool.
again these should be treated as no more than suggestions until verified oterwise
http://www.swissbioisostere.ch/
http://www.swissadme.ch/
there is also SMARTCYP to predict CYP450 metabolism sites
https://smartcyp.sund.ku.dk/mol_to_som

some steroid stuff... 4-Cl analogs of norboletone/THG

turns out "2c-b-fentanyl" (??) compound is a real thing, cayman will (not) sell it to you if you are dumb enough to spend $75 on a single milligram of some total unknown. no word as of yet on if it is psychedelic though. i would bet it would be at least moderately active as a mu opioid ligand & totally inactive as a psych (due to the general rule of thumb for PEAs being that primary amines are needed) but damn, talk about turning a (precursor to a) safe drug into a deadly one :/
i can see it now... "super potent psychedelic heroin vapes: the latest drug threat????"

possible pcp analogs without piperidine:
a. 1-adamantyl
b. 2-adamantyl
c. cyclohexyl
d. isopropyl
e. cyclopentyl
f. 3-methylpyrrolidine
[edit]
Midaflur, a wierd sedative?

It looks like... well, I've never seen a drug quite a sstrange as it, that's for sure.

Fenpentadiol, a "swiss army knife"?
In three articles the properties of a new psychotropic agent from the series of araliphatic alcohols — phenpentanediol (CXV) — were described (477-479). It is not easy to place this substance anywhere in the pharmacodynamic system of psychotropic agents: on the one hand it potentiates the barbiturate narcosis, on the other it increases motility and exploratory activity in mice and potentiates the effects of amphetamine.

Its close relative phenaglycodol is a sedative like meprobabmate. Only 3 heteroatoms and no N!
also if you want people to be able to understand the structures you post, try either using iupac names or posting images of said compounds... to me SMILES is all greek & i have to plug every one into chemdraw to figure out what you are talking about.
also keep in mind STP is not a replacement for "real" pharmacological data
but in the interest of other cool tools, there is also supposedly a bioisostere replacement tool as well as a ADME prediction tool.
again these should be treated as no more than suggestions until verified oterwise
http://www.swissbioisostere.ch/
http://www.swissadme.ch/
there is also SMARTCYP to predict CYP450 metabolism sites
https://smartcyp.sund.ku.dk/mol_to_som

some steroid stuff... 4-Cl analogs of norboletone/THG

turns out "2c-b-fentanyl" (??) compound is a real thing, cayman will (not) sell it to you if you are dumb enough to spend $75 on a single milligram of some total unknown. no word as of yet on if it is psychedelic though. i would bet it would be at least moderately active as a mu opioid ligand & totally inactive as a psych (due to the general rule of thumb for PEAs being that primary amines are needed) but damn, talk about turning a (precursor to a) safe drug into a deadly one :/
i can see it now... "super potent psychedelic heroin vapes: the latest drug threat????"

possible pcp analogs without piperidine:
a. 1-adamantyl
b. 2-adamantyl
c. cyclohexyl
d. isopropyl
e. cyclopentyl
f. 3-methylpyrrolidine
[edit]
Midaflur, a wierd sedative?

It looks like... well, I've never seen a drug quite a sstrange as it, that's for sure.

Fenpentadiol, a "swiss army knife"?
In three articles the properties of a new psychotropic agent from the series of araliphatic alcohols — phenpentanediol (CXV) — were described (477-479). It is not easy to place this substance anywhere in the pharmacodynamic system of psychotropic agents: on the one hand it potentiates the barbiturate narcosis, on the other it increases motility and exploratory activity in mice and potentiates the effects of amphetamine.

Its close relative phenaglycodol is a sedative like meprobabmate. Only 3 heteroatoms and no N!
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Could you explain how Fenpentadiol potentiates amphetamine?
AcryloylFentanyl was a winner, so why not:
3M-AcryloylFentanyl
 swisstargetprediction.ch
Beta-OH-AcryloylFentanyl
swisstargetprediction.ch
Beta-OH-AcryloylFentanyl
 swisstargetprediction.ch
And Alpha-Methyl-AcryloylFentanyl
swisstargetprediction.ch
And Alpha-Methyl-AcryloylFentanyl
 swisstargetprediction.ch
Last but not least,
swisstargetprediction.ch
Last but not least,
1[2,6 CycloHexyl]-AcryloylFentanyl
 swisstargetprediction.ch
swisstargetprediction.ch
In my souvenirs, this:
 swisstargetprediction.ch
was being sold as 4-CDC, up for women 40+, good for their libido, or maybe it was the 4-Chloro, which would resemble Fenpentadiol a lot.
swisstargetprediction.ch
was being sold as 4-CDC, up for women 40+, good for their libido, or maybe it was the 4-Chloro, which would resemble Fenpentadiol a lot.
Edit: The 4-Chloro is alnost inactive.
Another Dopaminergic chemical with CB activity (this one of my own invention):
CN1C(C1C1=CC=CC=C1)C1=CC2=C(S1)C=CC=C2

 swisstargetprediction.ch
swisstargetprediction.ch
And talking of dissociatives, I like zis one:
CC1CCCC1(N1CCCC1)C1=CC=CC=C1

I committed to naming it
IZNOGOOD
As
He'll be seen flying on his magic carpet..
 swisstargetprediction.ch
swisstargetprediction.ch
Adding a methyl group on the cyclopentyl seems to make up for the size of it, as in:
 swisstargetprediction.ch
swisstargetprediction.ch
AcryloylFentanyl was a winner, so why not:
3M-AcryloylFentanyl
SwissTargetPrediction
SwissTargetPrediction
SwissTargetPrediction
1[2,6 CycloHexyl]-AcryloylFentanyl
SwissTargetPrediction
In my souvenirs, this:
SwissTargetPrediction
Edit: The 4-Chloro is alnost inactive.
Another Dopaminergic chemical with CB activity (this one of my own invention):
CN1C(C1C1=CC=CC=C1)C1=CC2=C(S1)C=CC=C2

SwissTargetPrediction
And talking of dissociatives, I like zis one:
CC1CCCC1(N1CCCC1)C1=CC=CC=C1

I committed to naming it
IZNOGOOD
As
He'll be seen flying on his magic carpet..
SwissTargetPrediction
Adding a methyl group on the cyclopentyl seems to make up for the size of it, as in:
SwissTargetPrediction
Last edited by a moderator:
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,993
nope. It's a mystery from an age in pharmacy where people threw compounds against the wall and saw what stuck.Could you explain how Fenpentadiol potentiates amphetamine?
Adding a methyl group on the cyclopentyl seems to make up for the size of it
journal references? I was always taught the cyclohexyl ring is kind of scared as such, reduction to a cyclopentyl or enlargement to the cycloheptyl doomed activity.

a benzomorphan

the trimer of piperidine produced from reaction of N-chloropiperidine formed from interaction of piperidine & chlorinating oxidisers like hypochlorite bleach, chlorine gas, trichlorotriazine etc
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
It threatens discovery to rely on what's already known.nope. It's a mystery from an age in pharmacy where people threw compounds against the wall and saw what stuck.
Hopefully some mad scientist is finding everything chemically safe just for form and testing it on a large pool of research specimens.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
The perfect cardiotoxin, I present to you:

CNC1C2CCCC2CC1C1=CC=C2C=CC=CC2=C1
 swisstargetprediction.ch
swisstargetprediction.ch
5HT2B and an out of the roof non-anaesthetic related HERG activity! :D Let's call this one Moliere, die on the scene..
Full serotonin 6 agonism:
8-Methyl-DMT

CN(C)CCC1=C(C)NC2=C1C=CC=C2
 swisstargetprediction.ch
swisstargetprediction.ch
It seems serotonin 6 is a regulator of Alzheimer development in elderly brains, here are some candidates: (it's quite hard to find non-5HT2b and orally active ones, that son't cause too many alterations of the senses with enough 5HT6, and a duration long enough for a day/half a day.)

CC(C)N(C)CCC1=C(C)NC2=C1C=CC=C2
 swisstargetprediction.ch
swisstargetprediction.ch

CC(CC1=C(C)NC2=C1C=CC=C2)N1CCCC1
 swisstargetprediction.ch
swisstargetprediction.ch

CC(C)N(CCC1=C(C)NC2=C1C=CC=C2)CC=C
 swisstargetprediction.ch
swisstargetprediction.ch
Or a combination of NC1CC2=C(C1)C=CC=C2 http://swisstargetprediction.ch/result.php?job=1013218982&organism=Homo_sapiens or NC1CCCC1C1=CC=CC=C1 http://swisstargetprediction.ch/result.php?job=1091801380&organism=Homo_sapiens

With
8-Methyl-DMT

CN(C)CCC1=C(C)NC2=C1C=CC=C2
Like 20 mg MAOI and 5 mg 8M-DMT.
Let's call it Dazimer, in I am.
Edit: Thanks for merging, it's a real hassle on my mobile phone.

CNC1C2CCCC2CC1C1=CC=C2C=CC=CC2=C1
SwissTargetPrediction
5HT2B and an out of the roof non-anaesthetic related HERG activity! :D Let's call this one Moliere, die on the scene..
Full serotonin 6 agonism:
8-Methyl-DMT

CN(C)CCC1=C(C)NC2=C1C=CC=C2
SwissTargetPrediction
It seems serotonin 6 is a regulator of Alzheimer development in elderly brains, here are some candidates: (it's quite hard to find non-5HT2b and orally active ones, that son't cause too many alterations of the senses with enough 5HT6, and a duration long enough for a day/half a day.)

CC(C)N(C)CCC1=C(C)NC2=C1C=CC=C2
SwissTargetPrediction

CC(CC1=C(C)NC2=C1C=CC=C2)N1CCCC1
SwissTargetPrediction

CC(C)N(CCC1=C(C)NC2=C1C=CC=C2)CC=C
SwissTargetPrediction
Or a combination of NC1CC2=C(C1)C=CC=C2 http://swisstargetprediction.ch/result.php?job=1013218982&organism=Homo_sapiens or NC1CCCC1C1=CC=CC=C1 http://swisstargetprediction.ch/result.php?job=1091801380&organism=Homo_sapiens

With
8-Methyl-DMT

CN(C)CCC1=C(C)NC2=C1C=CC=C2
Like 20 mg MAOI and 5 mg 8M-DMT.
Let's call it Dazimer, in I am.
Edit: Thanks for merging, it's a real hassle on my mobile phone.
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Does everyone here assume, across this whole visible universe, the greatest concentration of diverse molecule types is here on earth? Even if in tiny pockets.
It is kind of like asking if you believe in life on other planets, unless there is some compact quantum form of natural phenomenon displaying many permutations at once which I haven't learned about.
I like to think, far out there, the periodic table works differently so the number rises so drastically with what you can do in any one place, the point is moot
Entertaining the 'No life elsewhere and no aliens' theory - this, the Earth alone is the pinnacle epicenter of all molecular designs of carbon(?)
It is kind of like asking if you believe in life on other planets, unless there is some compact quantum form of natural phenomenon displaying many permutations at once which I haven't learned about.
I like to think, far out there, the periodic table works differently so the number rises so drastically with what you can do in any one place, the point is moot
Entertaining the 'No life elsewhere and no aliens' theory - this, the Earth alone is the pinnacle epicenter of all molecular designs of carbon(?)
- Status
- Not open for further replies.


