serotoninsoldier
Greenlighter
- Joined
- Jun 10, 2017
- Messages
- 1
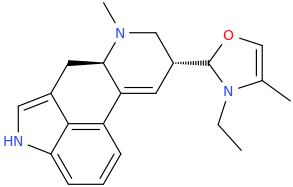
4‐(5‐fluoro‐2‐methoxy‐2,3‐dihydro‐1H‐inden‐1‐yl)‐6‐
methyl‐6,11‐
diazatetracyclo[7.6.1.02,7.012,16]hexadeca‐
1(16),2,9,12,14‐pentaene
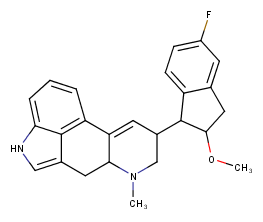
so i generated some fragments for lsd from the 5tvn pdb, then i grew that fragment until i reached this and the simulation said it had about the same affinity for 5ht2b as lsd. but idk it looks pretty bulky lol.
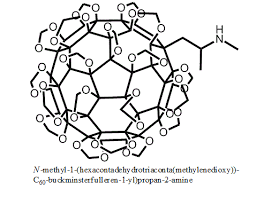
this one is prob better
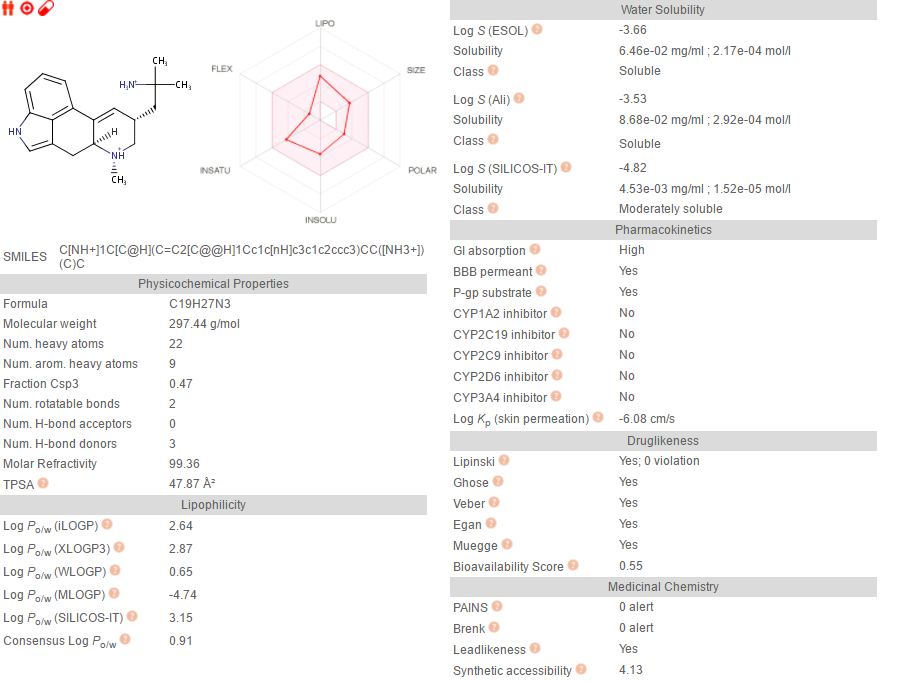
methyl‐6,11‐
diazatetracyclo[7.6.1.02,7.012,16]hexadeca‐
1(16),2,9,12,14‐pentaene

so i generated some fragments for lsd from the 5tvn pdb, then i grew that fragment until i reached this and the simulation said it had about the same affinity for 5ht2b as lsd. but idk it looks pretty bulky lol.
this one is prob better

Last edited: